The regeneration of severely damaged skin, say after serious burns, has taken another step toward reality as a team of investigators from the University of Calgary published exciting new data in understanding how skin heals, which could lead to drug treatments to vastly improve wound healing. Findings from the new study were published recently in Cell Stem Cell through an article titled “Distinct Regulatory Programs Control the Latent Regenerative Potential of Dermal Fibroblasts during Wound Healing.”
People who suffer severe burns or extensive skin injuries are often left to live with extreme scarring, disfigurement, and skin that feels chronically tight and itchy. That’s because the body’s healing processes have evolved to focus on preventing infection by quickly closing up wounds, rather than regenerating or restoring normal skin tissue. This new study offers new knowledge on why certain dermal cells are able to regenerate new skin, rather than disfiguring scar tissue.
“We identified a specific population of progenitor cells that reside within the dermis, the deep connective tissue of the skin,” explained senior study investigator Jeff Biernaskie, PhD, professor of stem cell biology, University of Calgary, faculty of veterinary medicine (UCVM), and the Calgary Firefighters Burn Treatment Society chair in skin regeneration and wound healing. “Progenitor cells are unique in that they are able to undergo cell division and generate many new cells to either maintain or repair tissues. Following injury, these dermal progenitors become activated, proliferate, and then migrate into the wound where they generate nearly all of the new tissue that will fill the wound, both scar, and regenerated tissue.”
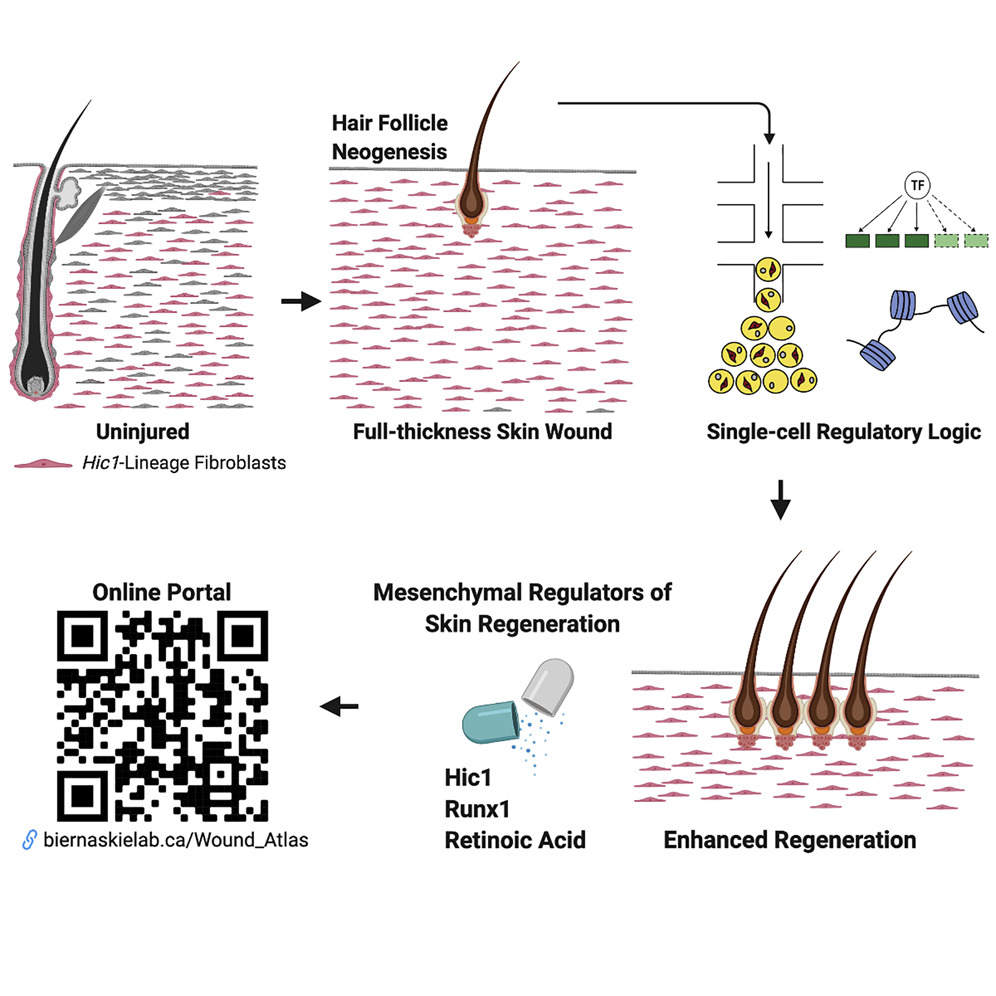
In the current analysis, the researchers used cutting-edge genomic techniques to profile thousands of individual cells at different times after injury and compared scar-forming versus regenerative zones within skin wounds.
“Remarkably, we found that although these cells come from the same cellular origin, different microenvironments within the wound activate entirely different sets of genes. Meaning, the signals found within ‘regenerative zones’ of the wound promote re-activation of genes that are typically engaged during skin development. Whereas, in scar-forming zones, these pro-regenerative programs are absent or suppressed and scar-forming programs dominate.”
“Using complementary fate-mapping approaches, we showed that hair follicle mesenchymal progenitors make limited contributions to wound repair,” the authors wrote. “In contrast, extrafollicular progenitors marked by the quiescence-associated factor Hic1 generated the bulk of reparative fibroblasts and exhibited functional divergence, mediating regeneration in the center of the wound neodermis and scar formation in the periphery. Single-cell RNA-seq revealed unique transcriptional, regulatory, and epithelial-mesenchymal crosstalk signatures that enabled mesenchymal competence for regeneration. Integration with scATAC-seq highlighted changes in chromatin accessibility within regeneration-associated loci.”
“What we’ve shown is that you can alter the wound environment with drugs, or modify the genetics of these progenitor cells directly, and both are sufficient to change their behavior during wound healing,” Biernaskie added. “And that can have really quite impressive effects on healing that includes regeneration of new hair follicles, glands, and fat within the wounded skin.”
Amazingly, this research offers critical insights into the molecular signals that drive scar formation during wound healing and it identifies a number of genetic signals that are able to overcome fibrosis and promote true regeneration of adult skin.
“This proof of principle is really important because it suggests that the adult wound-responsive cells do in fact harbor a latent regenerative capacity, it just simply needs to be unmasked,” Biernaskie concluded. “Now, we are actively looking for additional pathways that may be involved. Our hope is to develop a cocktail of drugs that we could safely administer in humans and animals to entirely prevent genetic programs that initiate scar formation in order to greatly improve the quality of skin healing.”



