Being able to grow working kidney tissue from human pluripotent stem cells (hPSCs) is essential to kidney regenerative medicine and could help accelerate medical treatments for kidney disease and restore kidney function.
The kidney forms normally in humans as a result of two building blocks—metanephric mesenchyme and ureteric bud (UB). Scientists had previously uncovered how to generate the first building block (metanephric mesenchyme) resulting in many components of the kidney from human stem cells.
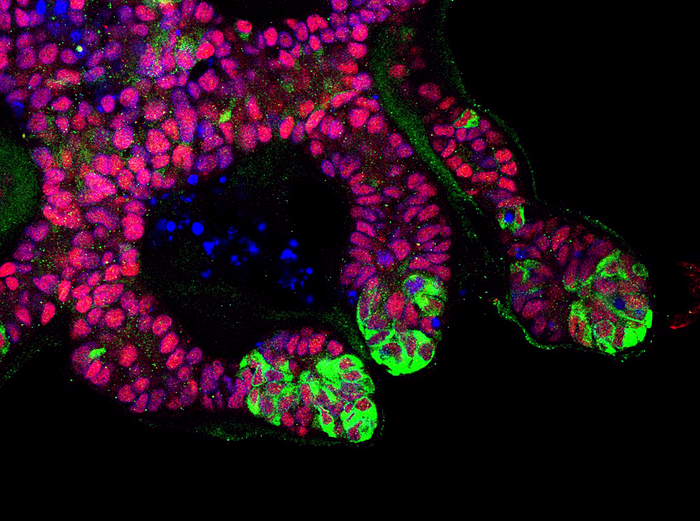
Now, researchers have developed a highly efficient method to generate the second building block (UB) which matures into the adult kidney collecting system. More specifically, they developed “highly efficient, serum-free differentiation of hPSCs into UB organoids and functional collecting duct cells.”
Further, they demonstrated features of interaction between the cells of these two building blocks, reproducing aspects of interaction that normally occur when the kidney develops.
In addition, for the first time ever, the Bonventre laboratory developed human cell lines of principal and intercalated cell lines, the two cell lines that make up the last urine processing component of the kidney. This research could help investigators test new therapies for treating kidney diseases that affect the collecting system. These include many congenital abnormalities of the kidney and urinary tract, including polycystic kidney disease.
This work is published in Nature Biotechnology in the paper, “Human ureteric bud organoids recapitulate branching morphogenesis and differentiate into functional collecting duct cell types.”
Kidney disease affects one in nine adults globally and the incidence of kidney failure is steadily rising around the world.
“We have developed a highly efficient way to generate a key component of kidney tissue responsible for maintaining many blood chemistries and critically important for development of the kidney. In addition, we have created, for the first time, human kidney cells that can be used to advance new drugs, aid in the investigation of inherited and acquired disorders, and improve our understanding of how the kidney develops and controls metabolic balance in the body,” said Joseph Bonventre, MD, PhD, chief of the renal unit and founding chief of the engineering in medicine division at the Brigham and Women’s Hospital in Boston.
How did they do it? The authors wrote that “hPSCs are first induced into pronephric progenitor cells at 90% efficiency and then aggregated into spheres with a molecular signature similar to the nephric duct.” In a three-dimensional matrix, the spheres form ureteric bud organoids. At later stages, they wrote, the ureteric bud organoids differentiate into collecting duct organoids, which contain >95% collecting duct cell types. This was determined through single-cell RNA sequencing. These collecting dust epithelia demonstrate renal electrophysiologic functions.
“Ultimately, with the ability to now generate both components responsible for making functional kidney tissue, this work provides a major step forward in the quest to replace renal function in patients with kidney failure or perhaps, in the future, generate a kidney in a dish.”