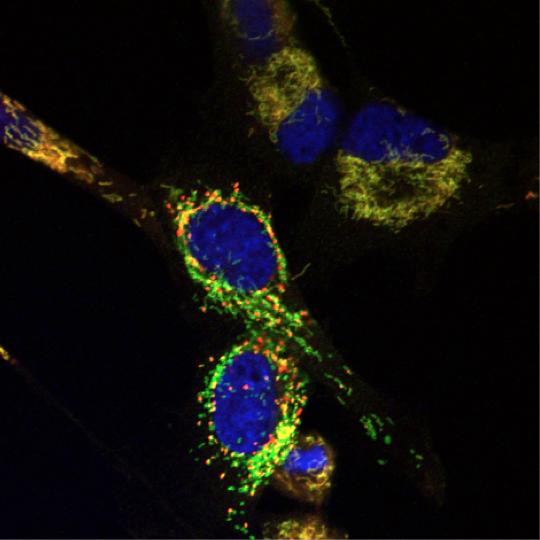
Brain tumor cells after treatment with Y100, which causes depolarization of mitochondria and the appearance of mitochondrial “hot spots” (red). [Yolanda Sanchez/Norris Cotton Cancer Center]
A team led by scientists at Dartmouth's Norris Cotton Cancer Center says it has devised a strategy to target cancer cells while sparing normal cells. This approach capitalizes on the fact that processes that allow a cell to form a tumor, such as loss or mutation of the tumor suppressor neurofibromin 1 (NF1), also expose vulnerabilities in the tumor cell that are absent in normal cells. These vulnerabilities are known as the “Achilles heel” of cancer cells.
Although much is known about the mutations that cause a cell to become malignant, little is known about the vulnerabilities of cells with these mutations. The researchers published their finding (“Exploiting Mitochondrial and Metabolic Homeostasis as a Vulnerability in NF1 Deficient Cells”) in Oncotarget.
“…we developed and conducted a synthetic lethality screen to discover molecules that target yeast lacking the homolog of NF1, IRA2. One of the lead candidates that was observed to be synthetic lethal with ira2Δ yeast is Y100,” write the investigators. “Here, we describe the mechanisms by which Y100 targets ira2Δ yeast and NF1-deficient tumor cells. Y100 treatment disrupted proteostasis, metabolic homeostasis, and induced the formation of mitochondrial superoxide in NF1-deficient cancer cells.”
Mutation of the tumor suppressor, NF1 or loss of the NF1 protein is a possible cause of aggressive neurological cancers, including glioblastoma (GBM), and has also been observed in lung adenocarcinoma and ovarian cancer among other sporadic cancers. Led by Norris Cotton Cancer Center's Yolanda Sanchez, Ph.D., the multi-institutional research team screened thousands of drug-like compounds for ones that would kill the NF1-deficient cells while sparing the wild-type (normal) cells and sorted out the lead compounds that were successful in doing so.
One of the lead candidates that was observed to be lethal with this particular mutation is called Y100.
“In [the Oncotarget] paper, we describe the mechanisms by which one of our top leads, Y100, targets NF1-deficient cells,” explains Dr. Sanchez. “Mutations that drive cells to become malignant, including loss of the tumor suppressor NF1, rewire cells' metabolism, which makes them uniquely sensitive to a process called oxidative stress. Our data so far suggest that Y100 can exploit this vulnerability in cells that lack the NF1 tumor suppressor.”
The team hypothesizes that the use of Y100 and molecules with related mechanisms of action represent a feasible therapeutic strategy for targeting NF1 deficient cells.
“Our long-term objective is to work with our board of scientific and clinical advisors to design Phase 0/I trials with agents that are efficacious at shrinking the tumors in 'avatar' models,” continues Dr. Sanchez. “In order to test the efficacy of Y100 against GBM tumors in whole organisms, we first need to examine the toxicity of Y100. To test the efficacy of Y100 we will use 'avatars,' which are mice carrying identical copies of patients' GBM tumors. When we identify the cellular target of Y100, then we can find additional inhibitors or drugs to test in the avatar models.”