Mutations in the gene CSF-1R have been linked to a rare form of dementia called leukoencephalopathy, which may help develop therapeutics for Alzheimer’s disease.
These findings are reported in the article, “Attenuated CSF-1R signaling drives cerebrovascular pathology,” published in EMBO Molecular Medicine.
Commenting on the clinical significance of the findings, Colin Doherty, MD, FRCPI, FFESM, professor of epilepsy, Trinity College, said: “It is absolutely critical that we focus our research endeavors on identifying the underlying cause of neurodegenerative conditions. Studies like these will pave the way for better clinical management of our patients and hopefully new medicines to treat the condition.”
Alzheimer’s disease is the fourth leading cause of death worldwide. Currently about 36 million people suffer from Alzheimer’s disease or related dementias globally and there are no approved medicines to prevent the progression of the disease. Pathologies related to blood vessels in the brain occur in about 80% patients with Alzheimer’s disease and are poorly understood.
“What we sought to do in our study was to examine a very rare form of brain disease called leukoencephalopathy with very similar characteristics to Alzheimer’s disease. We’ve defined the genetic cause of this condition,” said Mathew Campbell, PhD, associate professor at Trinity and senior author on the study.
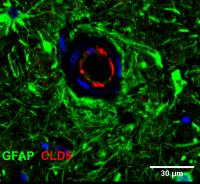
“We’ve discovered two new mutations in a gene called colony stimulating factor-1 receptor or CSF-1R. These mutations have led to a loss of function in the white blood cells that circulate throughout your body. And we’ve now tied this loss of function to damage at the blood vessels of the brain which leads to dementia,” said Conor Delaney, PhD, postdoctoral research fellow.
Adult-onset leukoencephalopathy is characterized by swollen axons, pigmented glia, and the building up of amyloid plaques on the walls of the arteries in the brain. The condition manifests initially with psychiatric and behavioral changes in patients followed by a rapid progression of dementia in the third or fourth decade of life. While the condition is very rare, it is devastating for affected families.
Scientists earlier believed leukoencephalopathy was caused by immune cells within the brain called microglia because the disease pathology involves degeneration of the white matter of the brain.
The current study identified two families with different mutations located in the enzymatically active region of the CSF-1R gene. The protein product of this gene acts as the receptor for two related ligands: colony stimulating factor-1 (CSF-1) and interleukin-34 (IL-34). CSF-1R function is critical for the activation of microglia and macrophages—white blood cells that engulf and destroy aberrant material such as bacteria or cellular debris.
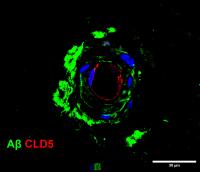
The study showed that loss of CSF-1R signaling disrupts the blood brain barrier and decreases the capacity of peripheral macrophages to engulf material without affecting the function of microglia. When CSF-1R function is compromised, macrophages cannot zero in on amyloid plaques effectively.
“This was fundamentally a translational research project, where data obtained from patient samples critically informed the direction of our preclinical studies. Our findings have shed light on a novel mechanism of neurodegeneration that may ultimately teach us more about common forms of dementia,” said Campbell.
The authors also showed that the molecular crosstalk between cells lining the blood vessels (endothelial cells) and microglial cells remodel the intercellular interactions of the blood brain barrier and the loss of CSF-1R function in patients and preclinical model animals, damages the blood brain barrier.
This suggests that regulating the integrity of the blood brain barrier and the recruitment of macrophages to the brain are therapeutically relevant to leukoencephalopathy and other Alzheimer’s-like dementias.
“We’ve identified potential therapeutic targets that could benefit both this rare disease leukoencephalopathy, and also much more common forms of dementia like Alzheimer’s disease,” said Campbell.



