For those who have suffered through a norovirus infection, commonly referred to as the “stomach flu,” the need for an effective vaccine does not really need explaining. But efforts to develop a vaccine against human norovirus have been unsuccessful, in part due to the virus’s rapid evolution and large number of antigenically distinct genotypes. Now, a group from the University of North Carolina (UNC) at Chapel Hill have discovered an antibody that broadly inhibits multiple strains of pandemic norovirus, a major step forward in the development of an effective vaccine for the dreaded stomach virus.
The work describes, for the first time, the structure of the binding interaction between norovirus and a human antibody that may work against many strains. Published in Immunity, the paper is titled “Sera Antibody Repertoire Analyses Reveal Mechanisms of Broad and Pandemic Strain Neutralizing Responses after Human Norovirus Vaccination.”
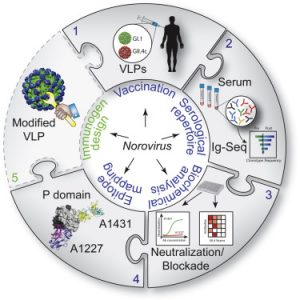
“In order to design an effective vaccine for norovirus, scientists needed to identify a neutralizing antibody that could work against many strains of the virus, as well as strains that will circulate in the future,” said Ralph Baric, PhD, professor at the UNC Gillings School of Global Public Health and senior author on the paper. “This information can now be used to build better human vaccines.”
The Centers for Disease Control and Prevention (CDC) notes that people with norovirus illness can shed billions of norovirus particles. And only a few virus particles can make other people sick. Symptoms include, primarily, vomiting and diarrhea with stomach pain. The vomiting can be many times in one day which can lead to dehydration, especially in young children, older adults, and people with other illnesses.
Although ∼60% of human norovirus outbreaks are caused by the GII.4 genotype, there are more than 30 known genotypes. These rapidly evolving RNA viruses elicit complex serological responses associated with previous exposure. In order to further understand the immune response to the infection, the team of researchers first established the GII.4 repertoire of antibodies present before and after vaccination and selected several for epitope and structural analysis. They found that the antibodies following vaccination are polarized and shaped by previous norovirus infection stating that the “antibody response was dominated by GII.4-specific antibodies that blocked ancestral strains or by antibodies that bound to divergent genotypes and did not block viral-entry-ligand interactions.” They went on to biochemically define the epitopes recognized by the antibodies with blockade activity to historical strains.
However, one antibody, A1431, showed broad blockade toward tested GII.4 strains and neutralized the pandemic strain. The authors write that “structural mapping revealed conserved epitopes, which were occluded on the virion or partially exposed, allowing for broad blockade with neutralizing activity.” The epitope of A1431 that has broad blockade breadth was determined by crystallography.
These results demonstrate that infection-derived and vaccine-elicited antibodies can exhibit broad blockade and neutralization against this prevalent human pathogen. The authors noted that “each of these antibody classes may contribute to vaccine-induced protection, and further studies and in vivo models are required to fully understand the interplay between the antibody targets and the physiological response.”


