Researchers at the University of Washington’s (UW) Institute for Protein Design (IPD) in Seattle say they have created a new protein that mimics the action of key immune regulatory interleukin 2 (IL-2), a potent anticancer drug and an effective treatment for autoimmune disease. However, its toxic side effects have limited its clinical usefulness.
In a paper (“De novo design of potent and selective mimics of IL-2 and IL-15”) recently published in Nature, the scientists report using computer programs to design a protein that they have shown in animal models to have the same ability to stimulate cancer-fighting T-cells as the naturally occurring IL-2, but without triggering harmful side effects. The achievement opens new approaches to the design of protein-based therapeutics for the treatment of cancer, autoimmune diseases, and other disorders, the researchers said.
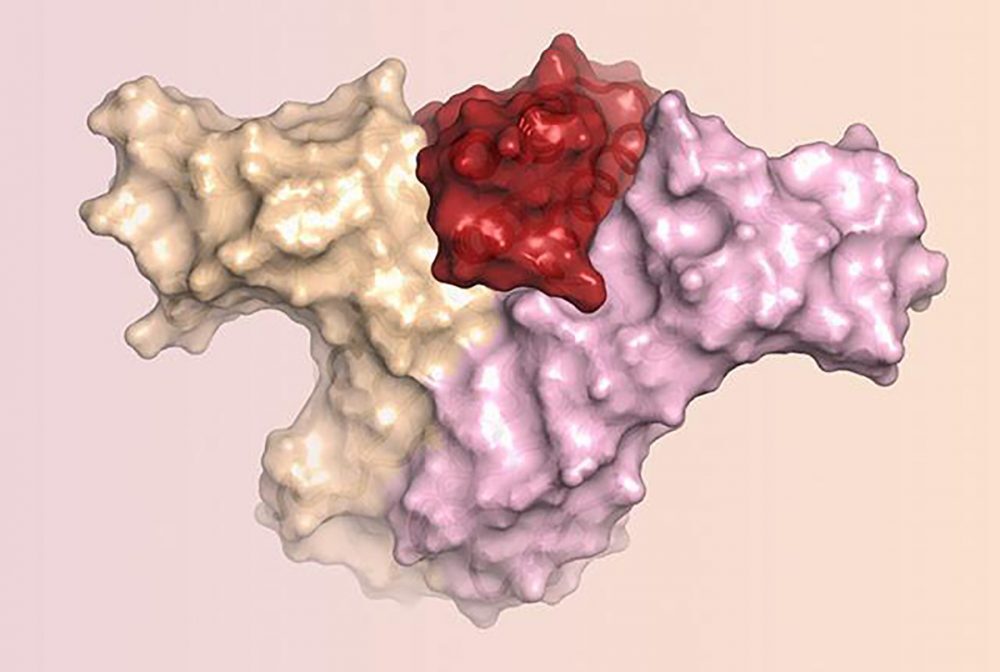
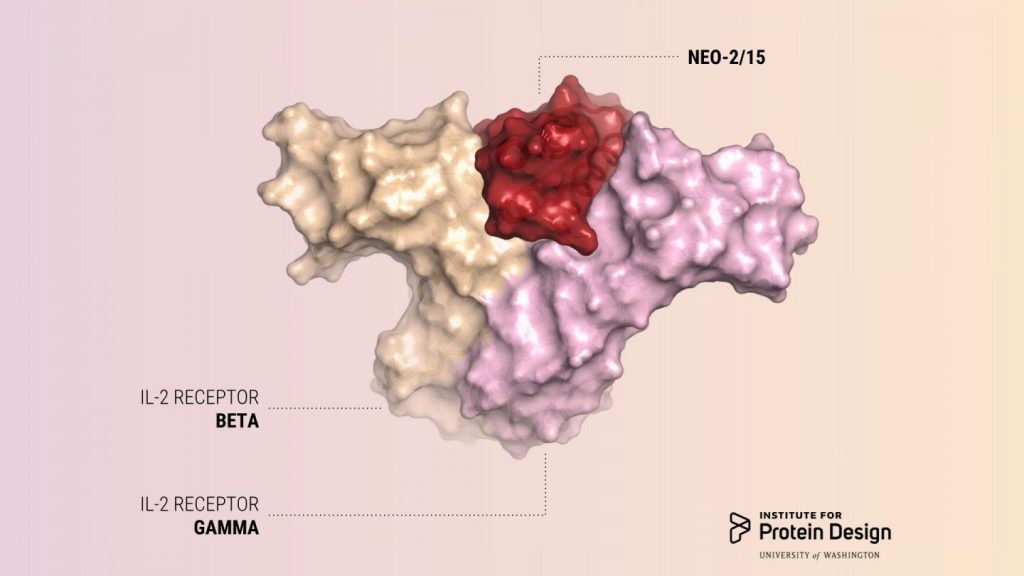
The new protein has been dubbed Neo-2/15 because, in addition to mimicking the effect of IL-2, the protein can also mimic the effect of another interleukin, IL-15, which is being studied as another possible anticancer immunotherapy.
“We describe a de novo computational approach for designing proteins that recapitulate the binding sites of natural cytokines, but are otherwise unrelated in topology or amino acid sequence. We use this strategy to design mimics of the central immune cytokine interleukin-2 (IL-2) that bind to the IL-2 receptor βγc heterodimer (IL-2Rβγc) but have no binding site for IL-2Rα (also called CD25) or IL-15Rα (also known as CD215). The designs are hyper-stable, bind human and mouse IL-2Rβγc with higher affinity than the natural cytokines, and elicit downstream cell signaling independently of IL-2Rα and IL-15Rα,” wrote the investigators.
“Crystal structures of the optimized design neoleukin-2/15 (Neo-2/15), both alone and in complex with IL-2Rβγc, are very similar to the designed model. Neo-2/15 has superior therapeutic activity to IL-2 in mouse models of melanoma and colon cancer, with reduced toxicity and undetectable immunogenicity. Our strategy for building hyper-stable de novo mimetics could be applied generally to signaling proteins, enabling the creation of superior therapeutic candidates.”
“People have tried for 30 years to alter IL-2 to make it safer and more effective, but because naturally occurring proteins tend not to be very stable, this has proved to be very hard to do,” said a lead author of the paper, Daniel-Adriano Silva, PhD, an IPD biochemist. “Neo-2/15 is very small and very stable. Because we designed it from scratch, we understand all its parts, and we can continue to improve it making it even more stable and active.”
“Neo-2/15 has therapeutic properties that are at least as good as or better than naturally occurring IL-2, but it was computationally designed to be much less toxic,” added another lead author, Umut Ulge, MD, an internal medicine physician and IPD biochemist.
IL-2 has been used as a last-ditch treatment for cancer patients with no other therapeutic options. For some patients with advanced melanoma or renal cell carcinoma, IL-2 can achieve cure rates as high as 7%. Its use, however, is limited because it can be given safely only to the healthiest patients and only in intensive-care units at specialized medical centers.
IL-2 acts on two kinds of immune cells by binding to receptors on the cells’ surface. The effect IL-2 has on a cell’s behavior depends in large part on the number and nature of these receptor interactions. Natural IL-2 can activate cells with beta and gamma receptors responsible for antitumor activity. However, natural IL-2 preferentially binds to another kind of immune cell which has alpha receptors in addition to beta and gamma receptors. These cells cause side effects like severe toxicity and immunosuppression. To date, all approved IL-2 therapies unfortunately cause preferential activation of these off-target cells.
The new protein does not preferentially bind to the harmful cells. This molecule enables activation of on-target tumor-fighting cells without preferentially activating the off-target cells responsible for toxicity and immunosuppression, according to the researchers.
The finding shows that designing proteins from scratch can lead to bio-superior molecules with enhanced therapeutic properties and lesser side effects for virtually any biological molecule whose structure is known or can be predicted, said lead researcher and institute director David Baker, a UW School of Medicine professor of biochemistry and a Howard Hughes Medical Institute investigator.
To design a cancer-fighting protein that would not cause these side effects, the researchers used a computer program developed in the Baker lab called Rosetta. The team designed their protein to have surfaces that would bind to and activate IL-2 receptor beta and gamma, but not the IL-2 receptor alpha, which is part of the harmful cells.
The scientists designed compact proteins to serve as scaffolds for holding the two binding sites in the proper position. Then they optimized the amino acid sequence of the best scaffolds. This effort resulted in a final compact protein that is completely different from natural IL-2. In laboratory and animal models it bound to IL-2 receptor beta and gamma, activated cancer-fighting immune cells, and slowed tumor growth. Because the designed protein had no binding site for the alpha receptor, effective doses of Neo-2/15 did not cause toxic side effects.