A critical component to advancing drug discovery for neurological diseases is obtaining neurons that can be used in research. Because human neurons can be hard to come by, alternative methods for creating them is an important area of study. Additionally, creating patient-specific neurons is a cornerstone of the future of personalized medicine.
A team of researchers from The Salk Institute in collaboration with the Institute for Stem Cell Biology and Regenerative Medicine at Stanford University and Baylor College of Medicine have shown that mouse cells that are induced to grow into nerve cells using a previously published method have molecular signatures matching neurons that developed naturally in the brain.
The study, titled “Global DNA methylation remodeling during direct reprogramming of fibroblasts to neurons” was published in eLife on January 15. The work opens the door for better ways to model an individual patient’s disease. This technique would enable researchers to study how neurological conditions develop, as well as to test new therapies. The new technology also could help to advance research into gene therapies that are derived from a patient’s own cells.
[Wernig lab/Stanford University]
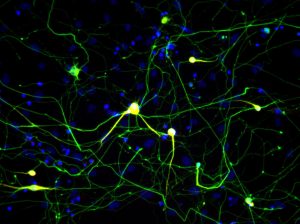
Fibroblasts, which make up most of the connective tissue in animals and play an important role in wound healing, were used in this study. The authors wanted to determine whether the fibroblasts that were transformed into neurons accurately corresponded to neurons that had grown naturally in the brain.
The technique for inducing the fibroblasts to grow into neurons with the matching epigenome was developed by Marius Wernig, MD, associate professor at Stanford and a co-senior author. With this method, making induced neuronal cells does not involve pluripotent intermediates. Instead, the cells are directly converted from fibroblasts to neurons.

[Salk Institute]
“An important question in cellular engineering is how to know the quality of your product,” says co-first author Chongyuan Luo, PhD, a postdoctoral fellow in Ecker’s lab. “If we’re making neurons from fibroblasts, we want to know how they compare with neurons in the brain. We are particularly interested in looking at these cells at the level of the epigenome.” Differences between the epigenomes of induced and naturally grown neurons could result in different features of induced neurons that might make them less accurate models of neuronal behavior.
Using a technique developed in the Ecker lab called MethylC-seq, the researchers looked at every place in the genome where chemical groups called methyl groups are attached. They confirmed that these induced neurons have epigenomes that match neurons in the brain.
“This research was done in mouse cells, but we plan to use the same technology to study induced neurons made with human cells,” explained Ecker.



