A two-ingredient antibacterial cocktail is more potent than either ingredient alone, even though one of the ingredients is meropenem, an antibiotic that induces multidrug resistance in bacteria. This surprising outcome depends on a twist—or rather, twist after twist after twist delivered by light-activated molecular nanomachines (MNMs).
MNMs were introduced by Rice University and Durham University scientists in 2017. In a recent study, these scientists, along with colleagues at Biola University and Texas A&M Health Science Center, used MNM-antibiotic combinations to kill bacteria that had evolved to exclude (and thereby resist) meropenem. Unlike resistant bacteria that deactivate internalized antibiotics, resistant bacteria that exclude antibiotics are vulnerable to MNM-antibiotic combinations. MNMs create holes through which once-excluded antibiotics may pass.
The new findings appeared in the journal ACS Nano, in an article titled, “Molecular Nanomachines Disrupt Bacterial Cell Wall, Increasing Sensitivity of Extensively Drug-Resistant Klebsiella pneumoniae to Meropenem.” The article suggests that MNMs could be useful in other contexts, that is, with other antibiotics and other pathogens.
If MNMs could restore the potency of conventional antibiotics against multidrug-resistant pathogens, they could prevent widespread suffering. “Superbugs could kill 10 million people a year by 2050, way overtaking cancer,” said Rice University’s James M. Tour, PhD, a professor of chemistry and one of the corresponding authors of the current paper. “These are nightmare bacteria; they don’t respond to anything.”
To counter such bacteria, Tour and colleagues deployed their MNMs, paddlelike structures that can be prompted to spin at three million rotations per second when activated with light. After attaching to bacteria and receiving light stimulation, the MNMs burrow through bacterial cell walls within minutes.
“We exposed extensively drug-resistant Klebsiella pneumoniae to light-activated MNMs and found that MNMs increase the susceptibility to meropenem,” wrote the article’s authors. “MNMs with meropenem can effectively kill K. pneumoniae that are considered meropenem-resistant.”
Using permeability assays and transmission electron microscopy, the authors examined the MNMs’ mechanism of MNM action. Essentially, MNMs disrupt the cell wall of extensively drug-resistant K. pneumoniae, exposing the bacteria to meropenem.
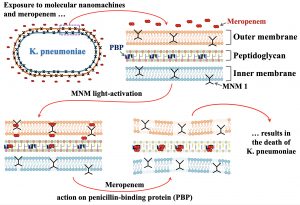
“Bacteria don’t just have a lipid bilayer,” Tour noted. “They have two bilayers and proteins with sugars that interlink them, so things don’t normally get through these very robust cell walls. That’s why these bacteria are so hard to kill. But they have no way to defend against a machine like these molecular drills, since this is a mechanical action and not a chemical effect.”
Bacterial colonies targeted with a small concentration of nanomachines alone killed up to 17% of cells, but that increased to 65% with the addition of meropenem. After further balancing motors and the antibiotic, the researchers were able to kill 94% of the pneumonia-causing pathogen.
Tour said the nanomachines may see their most immediate impact in treating skin, wound, catheter, or implant infections caused by bacteria—like Staphylococcus aureus, Klebsiella, or Pseudomonas—and intestinal infections. “On the skin, in the lungs, or in the gastrointestinal tract, wherever we can introduce a light source, we can attack these bacteria,” he asserted. “Or one could have the blood flow through a light-containing external box and then back into the body to kill blood-borne bacteria.”
“We are very much interested in treating wound and implant infections initially,” added Jeffrey D. Cirillo, PhD, a professor of microbial and molecular pathogenesis at Texas A&M and another coauthor of the current study. “But we have ways to deliver these wavelengths of light to lung infections that cause numerous mortalities from pneumonia, cystic fibrosis, and tuberculosis, so we will also be developing respiratory infection treatments.” Bladder-borne bacteria that cause urinary tract infections may also be targeted.


