
Eureka moments occur when the right elements suddenly fall into place. Such moments are always satisfying, but they are especially so when the “right” elements would have been deemed “wrong,” at least at first. Consider these two elements: metal nanocatalysts and cellular bioenergetics. Ordinarily, metal nanocatalysts drive reactions in industrial processes. But at Clene Nanomedicines, metal nanocatalysts are becoming relevant to biological processes.
Clene is using metal nanocatalysts to solve the energy problems that occur in aging cells and leave us vulnerable to disease. Clene’s approach involves two of the building blocks of energy in the cell: the oxidized and the reduced forms of nicotinamide adenine dinucleotide (NAD+ and NADH, respectively).
“In our 20s, NAD+ peaks,” notes Rob Etherington, president and CEO of Clene. “Then it declines in a linear fashion decade after decade.”
Clene believes that elevating NAD+ has therapeutic potential. To explore this idea, Clene has developed a metal nanocatalyst called CNM-Au8. CNM stands for Clean-Surfaced Nanocrystal, and “Au” is, of course, the chemical symbol for gold.
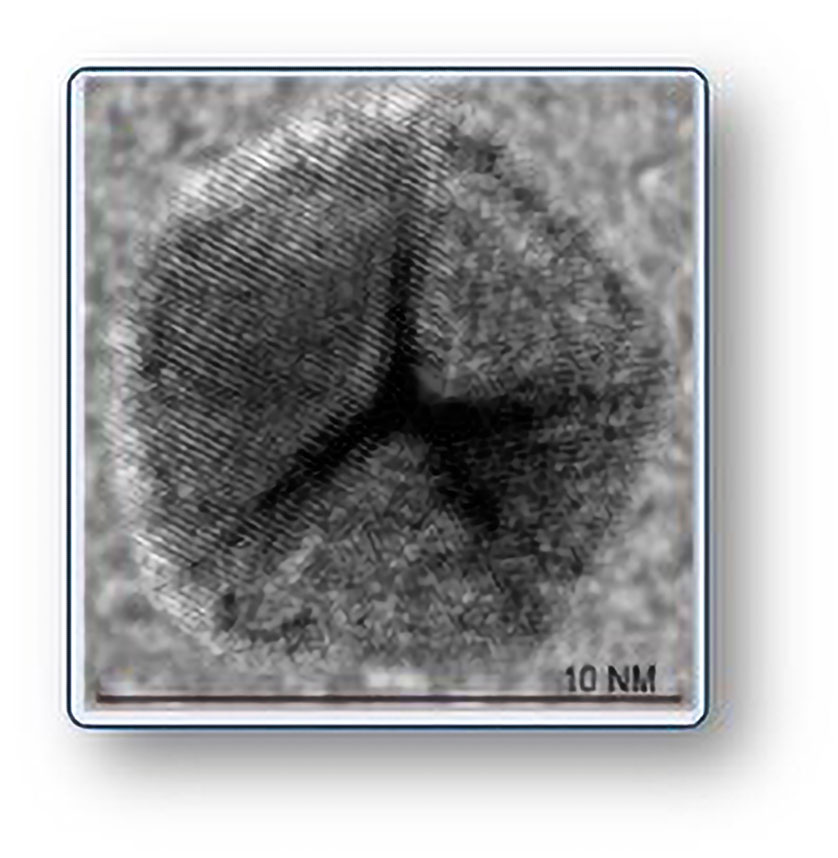
CNM-Au8’s nanocatalytic activity was assessed in a recent paper (Robinson et al. Sci. Rep. 2020; 10(1): 1936). This paper showed that CNM-Au8 stimulated and supported energy metabolic pathways in oligodendrocyte membranes, promoting remyelination of axons and recovery of behavioral functions in animal models of multiple sclerosis.
The paper’s authors, who included Clene-affiliated scientists, noted that one of the key reactions catalyzed by gold nanoparticles is the oxidation of NADH to the critical energetic co-factor, NAD+. “NAD+ and NADH are not only metabolic sensors of cellular energy levels, but also serve as the essential redox couple for ATP-generating reactions, oxidative phosphorylation and glycolysis,” the authors wrote. “In addition, NADH oxidation drives cellular respiratory and metabolic processes that play key roles in the energetically demanding process of myelination.”
A materials science–based approach
Clene applies plasma physics and materials science to engineer nanocrystal suspensions, and the nanocrystals—which consist of gold, silver, zinc, platinum, or alloys of these metals—are free of chemical surfactants or surface capping agents. For example, CNM-Au8 is made by an electrocrystallization process using pure gold wire, water, and sodium bicarbonate to create a suspension of clean, faceted nanocrystals. (The nanocrystals stay clean because they resist the accumulation of potentially toxic organic residues.)
CNM-Au8 is being studied in multiple clinical trials, including two trials for the treatment of multiple sclerosis (MS), a trial for the treatment of Parkinson’s, and two trials for the treatment of amyotrophic lateral sclerosis (ALS). Clene is also developing a zinc-silver nanoparticle (for antiviral and antibacterial applications), a topical gel polymer suspension of silver and zinc ions (for wound healing and burn treatment), and a platinum-gold nanoparticle (for oncology applications).
Convenient storage and administration
CNM-Au8 has a shelf life of at least 2.5 years, and it has “a pretty nice toxicology profile, as patients are only drinking nanocrystals of gold in water,” Etherington says. Patients drink a 60 mL bottle of CNM-Au8 once daily. This simple regimen can enhance key metabolic reactions and improve energy production in the neurons of the central nervous system.
To explain the benefits of using CNM-Au8 to catalyze energy production, Etherington offers a before-and-after scenario. Before: Diseased cells lack the energy to do their own housekeeping. After: Treated cells have the energy to attend to their own needs. Essentially, diseased cells suffer oxidative stress and are unable to prevent the accumulation of harmful byproducts. Treated cells have the energy to resist stressors, and they are better able to halt or repair neurodegeneration.
Clinical progress
In August, Clene released results from REPAIR-PD and REPAIR-MS, the first clinical studies of CNM-Au8 for the treatment of Parkinson’s disease and MS, respectively (13 patients were in REPAIR-PD, and 11 patients were in REPAIR-MS). “These were small target biomarker/target engagement studies,” Etherington details. “[They showed that] we’re significantly increasing brain energetic metabolism.”
The studies achieved a statistically significant increase in the ratio of NAD+ to NADH. They also demonstrated consistent brain target engagement and provided proof of mechanism.
Etherington expects a readout soon from a double-blind, placebo-controlled, Phase II trial in ALS patients. This trial will show whether the compound meaningfully improves muscle control in patients’ hands and legs. “ALS patients lose up to 40% of their muscle function in a year, so we’re seeing whether we can stabilize that loss or improve muscle function,” Etherington elaborates.
Clene’s nanomedicines have already shown improvements in functional measurements in trials involving ALS and MS patients. However, Etherington suggests that results from these trials should be regarded with caution. Functional improvements, he says, may reflect profound placebo effects.
Blinded data from the RESCUE-ALS study that was reported at the European Network to Cure ALS 2021 Annual Meeting showed a functional change in muscles. And data from the double-blind placebo-controlled VISIONARY-MS study that was presented at the Americas Committee for Treatment and Research in Multiple Sclerosis Forum 2021 showed improvements in the Multiple Sclerosis Functional Composite (MSFC) score.
“Patients saw consistent improvements across four scales of the MSFC score—the ability to see, fine motor movement of the fingers and hands, working memory, and the ability to walk quickly,” Etherington details. Registration endpoints at three, six, and nine months show improvements across the entire population of trial participants.
Untapped power
Clene Nanomedicine is based on the scientific work of founder and chief science officer Mark Mortenson. About 10 years ago, he developed an electrocrystallization process to build a nanosuspension system that can serve as an electron donor to living cells. The process is proprietary and forms the foundation for more than 130 patents.
Clene’s initial challenge was engineering. Eventually, the company got the particle size to a mere 13 nm—small enough to fit inside mitochondria. Clene then spent nearly six years working with animal models of neuron failure and remyelination diseases to prove its thesis.
Clene is especially proud of what it showed in the REPAIR trials. “We showed for the first time that a drug could treat energetic failure in the brains of neurodegenerative patients,” Etherington asserts. “I can’t underscore that enough. In the next year or so, key data will emerge proving whether a nanotherapeutic based around gold can drive, or return, energy into our central nervous system.”
Positive trial results would be “tremendously useful for patients,” he says. “ALS patients presently have only two drugs approved in the United States that offer some help, and patients generally suffer a devastating decline in neurological function.” Their postdiagnosis survival rate is three to five years. The situation for MS is similar. The approved drugs stabilize the disease but do not improve neurological function. Adding CNM-Au8 to the established drug regimen may help it do both.
“Our entire focus is to reach the conclusion of these studies to improve patients’ energetic metabolism,” Etherington insists. “There are additional multiple neurodegenerative diseases, both rare and common, that affect us as we age. If we can demonstrate improved brain neurometabolism, like when we are young, we may be able to return the cells to energetic homeostasis. That’s a huge idea.”
