In autoimmune diseases such as type 1 diabetes and multiple sclerosis, an organism mounts an energetically costly and misdirected inflammatory immune response against its own organs.
Multiple sclerosis is the most common autoimmune disease of the central nervous system where myelin, a protective insulating sheath surrounding nerve cells that aids the efficient transmission of electrical signals, is destroyed by the body’s own immune system, leading to a broad range of progressive symptoms including changes or loss of vision, numbness, tingling, incontinence, muscle spasticity, fatigue, tremors, and paralysis.
Studying mouse models of multiple sclerosis, scientists at the University of Geneva (UNIGE) have discovered exposure to cold redirects the body’s resources from the immune system to maintaining body heat, thereby decreasing the course of the disease.
The study is featured in an article in the journal Cell Metabolism titled, “Cold Exposure Protects from Neuroinflammation Through Immunologic Reprogramming.” The findings pave the way for utilizing a fundamental concept in biology of the allocation of bioenergy to treat autoimmune diseases.
“While this increase is undoubtedly multifactorial, the fact that we have an abundance of energy resources at our disposal may play an important but as yet poorly understood role in autoimmune disease development,” said Doron Merkler, PhD, professor at the department of pathology and immunology and the Centre for Inflammation Research at the UNIGE Faculty of Medicine and co-corresponding author of the paper.
Immune surveillance in a healthy organism, and more so during inflammation, involves the generation of millions of immune cells every day and is an energetically expensive ordeal. It is a widely accepted theory in biology, that an organism redirects resources from growth and reproduction to survival and maintenance mechanisms such as energy conversation and defense when exposed to adverse situations like a hostile, cold environment.
When exposed to cold, nerve endings signal blood vessels to squeeze and the body burns brown fat to increase the generation of heat and cut down on its dissipation.
“The defense mechanisms of our body against the hostile environment are energetically expensive and can be constrained by trade-offs when several of those are activated. The organism may therefore have to prioritize resource allocation into different defense programs depending on their survival values,” said Mirko Trajkovski, PhD, professor in the department of cellular physiology and metabolism and the Diabetes Centre at the Faculty of Medicine of the UNIGE, and lead author of the study. “We hypothesized that this can be of particular interest for autoimmunity, where introducing an additional energy-costly program may result in milder immune response and disease outcome. In other words, could we divert the energy expended by the body when the immune system goes awry?”
To answer this question, the researchers placed mice with experimental autoimmune encephalomyelitis (EAE), a model of human multiple sclerosis, in an environment where they gradually reduced the temperature to 10°C to allow the mice to acclimate to the low temperature.
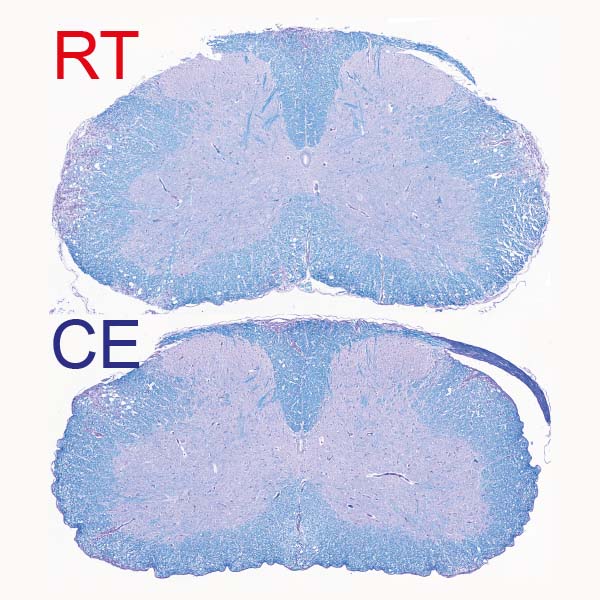
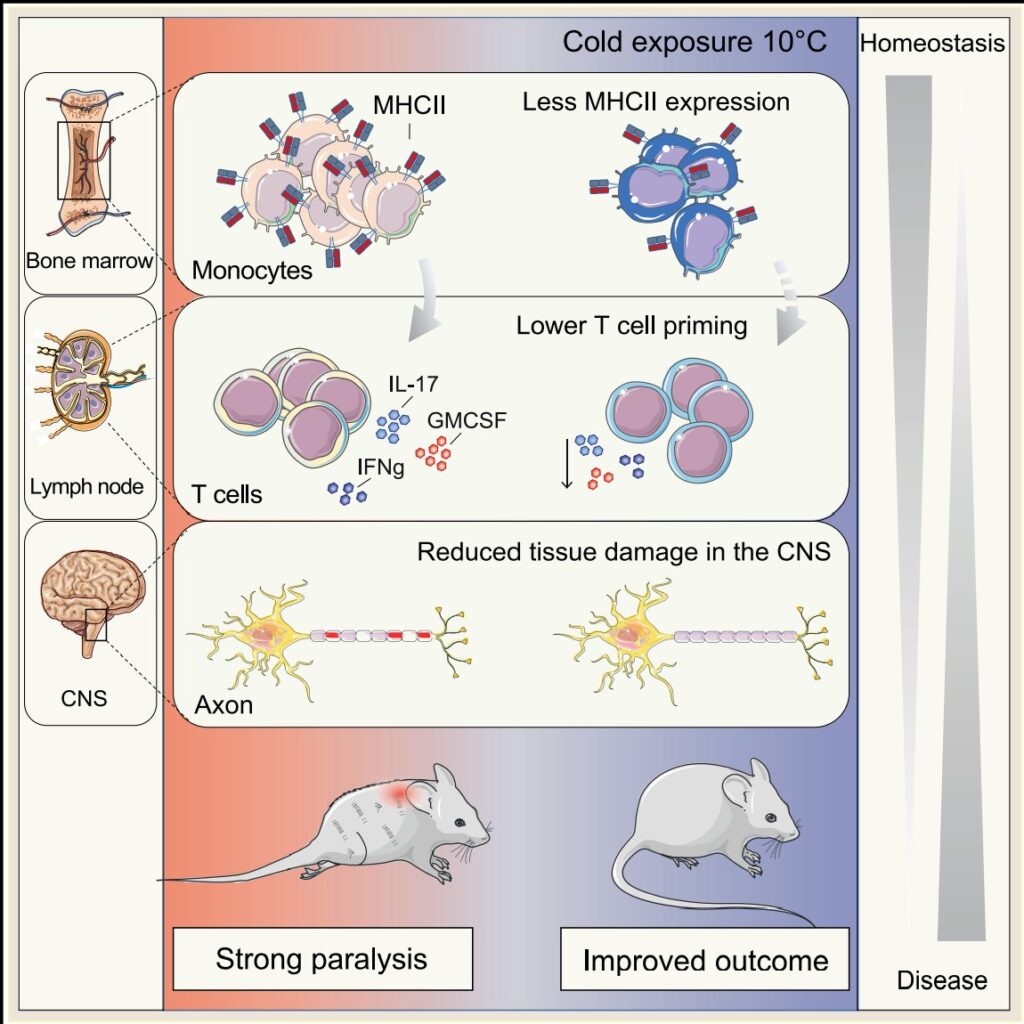
“After a few days, we observed a clear improvement in the clinical severity of the disease as well as in the extent of demyelination observed in the central nervous system,” said Merkler. “The animals did not have any difficulty in maintaining their body temperature at a normal level, but, singularly, the symptoms of locomotor impairments dramatically decreased, from not being able to walk on their hind paws to only a slight paralysis of the tail.”
Distinguishing between protein antigens that belong to the organism (self) versus those that belong to intruders such as pathogens (non-self) is a key facet of immune defense. Antigen-presenting cells chew up foreign proteins and present their fragments, much like mobile billboards, to inform immune cells such as T lymphocytes of the presence of external threats. The underlying malady in autoimmune diseases is the inability of immune cells to distinguish between antigens of the “self” and “non-self.”
“We show that cold modulates the activity of inflammatory monocytes by decreasing their antigen-presenting capacity, which rendered the T cells, a cell type with a critical role in autoimmunity, less activated,” said Trajkovski.
Specifically, the authors showed cold temperatures decrease major histocompatibility complex class II (MHCII) on monocytes under normal conditions and during inflammation, through their mouse model studies. This suppresses T-cell priming and pathogenicity. When monocytes were experimentally depleted through genetic manipulations or by treating mice with antibodies directed against monocytes, the cold-induced effects on T cells or experimental autoimmune encephalomyelitis was abolished.
“While the concept of prioritizing the thermogenic over the immune response is evidently protective against autoimmunity, it is worth noting that cold exposure increases susceptibility to certain infections. Thus, our work could be relevant not only for neuroinflammation, but also other immune-mediated or infectious diseases, which warrants further investigation,” added Trajkovski.
In their next steps, the researcher will try to understand whether these findings can be applied in clinical treatments of immune dysfunctions.



