Muscle mass decreases approximately 3–8% per decade after the age of 30 and this rate of decline is even higher after the age of 60, according to studies. This involuntary loss of muscle mass, strength, and function is a fundamental cause of and contributor to disability in older people. To overcome this loss, Salk Institute scientists are studying ways to accelerate the regeneration of muscle tissue, using a combination of molecular compounds that are commonly used in stem cell research.
Their findings are published in Nature Communications, in a paper titled, “In vivo partial reprogramming of myofibers promotes muscle regeneration by remodeling the stem cell niche.”
“Reprogramming of somatic cells to a pluripotent state by overexpressing the Yamanaka factors (Oct-3/4, Sox2, Klf4, and c-Myc [OSKM]) is a long and complex process,” wrote the researchers. “Cellular reprogramming is widely utilized for disease modeling in vitro. However, reprogramming in vivo induces tumor development. Our lab showed that partial reprogramming by short-term expression of reprogramming factors ameliorated aging hallmarks without tumor formation, opening a possible application of this approach in vivo.”
“Loss of these progenitors has been connected to age-related muscle degeneration,” explained Salk professor Juan Carlos Izpisua Belmonte, PhD, the paper’s senior author. “Our study uncovers specific factors that are able to accelerate muscle regeneration, as well as revealing the mechanism by which this occurred.”
The researchers have previously demonstrated factors that can rejuvenate cells and promote tissue regeneration in live animals, but the underlying mechanisms were not previously known.
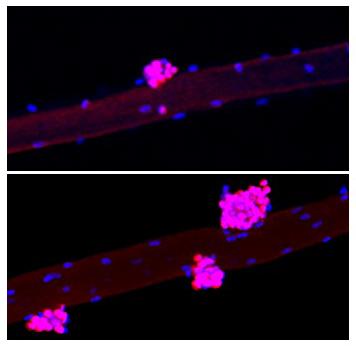
The researchers used two different mouse models to pinpoint the muscle stem-cell-specific or niche-specific changes following addition of Yamanaka factors.
In the myofiber-specific model, they found that adding the Yamanaka factors accelerated muscle regeneration in mice by reducing the levels of a protein called Wnt4 in the niche. In the satellite-cell-specific model, Yamanaka factors did not activate satellite cells and did not improve muscle regeneration, suggesting that Wnt4 plays a critical role in muscle regeneration.
This study could eventually lead to new treatments by targeting Wnt4.
“Our laboratory has recently developed novel gene editing technologies that could be used to accelerate muscle recovery after injury and improve muscle function,” Belmonte said. “We could potentially use this technology to either directly reduce Wnt4 levels in skeletal muscle or to block the communication between Wnt4 and muscle stem cells.”
Further research is needed before this approach can be applied in humans. However, the research provides insight into the underlying mechanisms related to muscle regeneration and growth and may lead to further studies and potential treatments to aid regeneration more effectively.