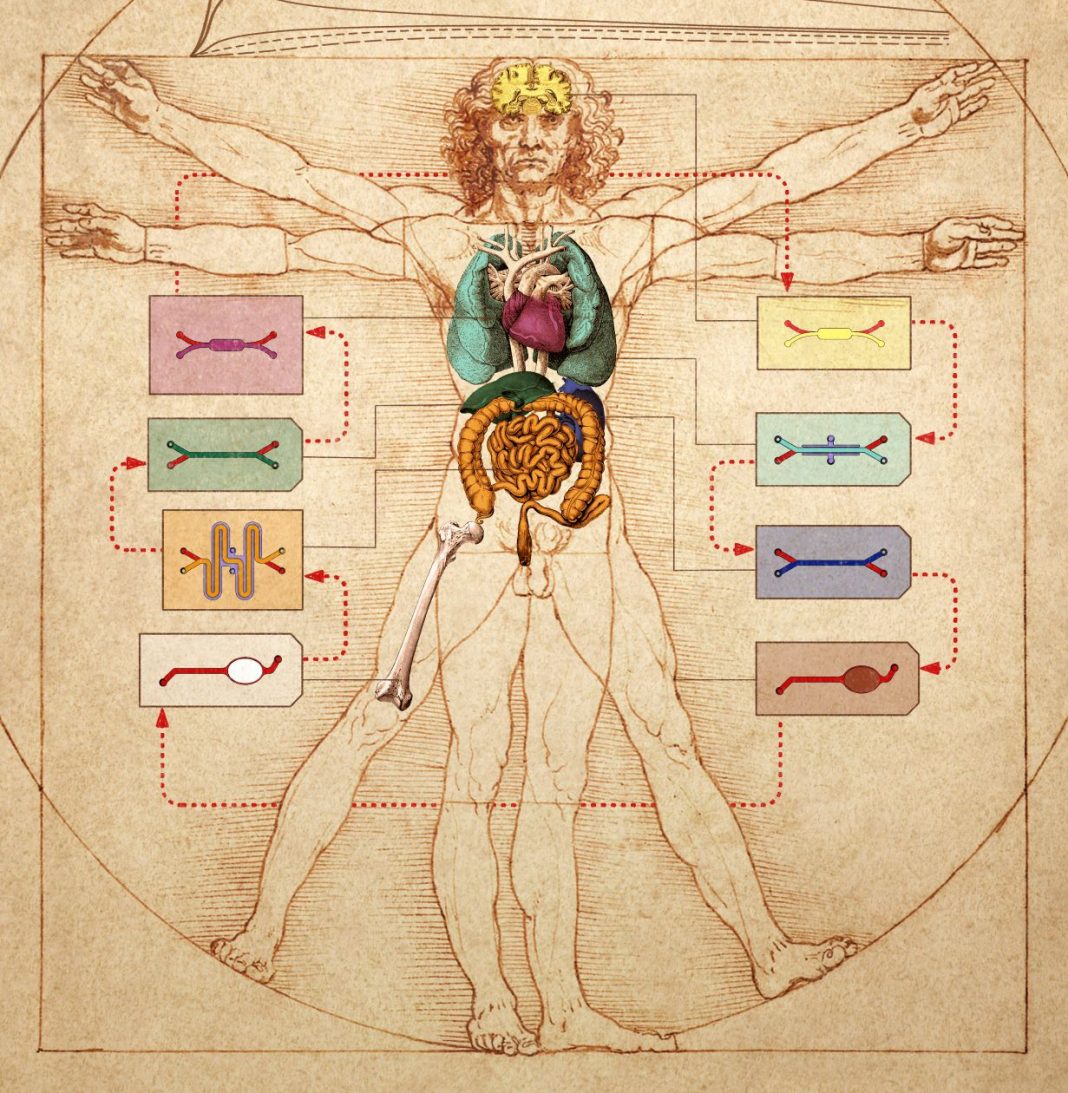
Historically, artists have been preoccupied with the deep connections between the human form and the rest of nature, and how these connections manifest in terms of proportion. Now, scientists are getting in on the act. At Harvard’s Wyss Institute, scientists have sketched the future of human physiology and pathophysiology modeling.
Instead of drawing on parchment or painting on canvas, the scientists have realized their vision in another medium—the microfluidic chip. They’ve demonstrated that they can transfer fluids between the vascular channels of many different types of organ-on-a-chip devices to mimic blood flow, thus engineering a body-on-a-chip device. Moreover, they’ve shown that the entire linked system, the body chip, can be used to represent how the human body responds to drugs.
The achievement is significant because it can help overcome a troublesome bottleneck in preclinical drug development. This bottleneck concerns the characterization of a drug’s pharmacokinetics (PK) and pharmacodynamics (PD). Typically, PK and PD studies require the use of animal models, which are inefficient and liable to give distorted views of human PK and PD responses. Also, animal studies pose ethical problems.
Replacing animal models with body chips could accelerate drug development, the Wyss Institute scientists suggest. “We need to become much more effective at setting the stage for drugs that are truly promising and rule out others that for various reasons are likely to fail in people,” explained Donald E. Ingber, MD, PhD, founding director of the Wyss Institute. “The modularity of our approach and availability of multiple validated organ chips for a variety of tissues for other human body chip approaches now allows us to develop strategies to make realistic predictions about the pharmacology of drugs much more broadly. Its future use could greatly increase the success rates of Phase I clinical trials.”
Ingber is the senior author of two back-to-back studies that appeared January 27 in Nature Biomedical Engineering. In the first article (“Robotic fluidic coupling and interrogation of multiple vascularized organ chips”), the Wyss team presents a highly modular body chip platform, which is enabled by an engineered “Interrogator” instrument that can culture up to 10 different organ chips, and sequentially transfer fluids between their endothelium-lined vascular channels to mimic normal human blood flow between the different organs of the body.
In the second article (“Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips”), the team uses a computational scaling method to translate data obtained from drug experiments involving three different types of fluidically linked organ chips to their respective organ dimensions in the real human body. The approach can quantitatively predict changes in drug levels over time, as well as organ-specific toxicities, that have been previously measured in human patients.
The Interrogator instrument enabled the team to culture, perfuse, and link many living human cultured tissues in a multi-organ chip system, as well as add and sample the medium in a programmable way, using the device’s robotic liquid transfer capabilities, while continuously monitoring tissue integrity with an integrated microscope.
“We used the robotic interrogator and a physiological multicompartmental reduced-order model of the experimental system to quantitatively predict the distribution of an inulin tracer perfused through the multi-organ human-body-on-chips,” the authors of the first article wrote. “The automated culture system enables the imaging of cells in the organ chips and the repeated sampling of both the vascular and interstitial compartments without compromising fluidic coupling.”
“We serially linked the vascular channels of eight different organ chips, including intestine, liver, kidney, heart, lung, skin, blood-brain barrier, and brain, using a highly optimized common blood substitute, while independently perfusing the individual channels lined by organ-specific cells,” said Richard Novak, PhD, a Wyss senior staff engineer who served as co-first-author on both studies. “The instrument maintained the viability of all tissues and their organ-specific functions for over three weeks and, importantly, it allowed us to quantitatively predict the tissue-specific distribution of a chemical across the entire system.”
In the second study, the team used the Interrogator instrument to support two different configurations of three different organ chips linked to each other and to a central arterio-venous (AV) fluid mixing reservoir that helped recapitulate life-like blood and drug exchange between the individual organs, while also providing a way to carry out blood sampling that would mimic blood drawing from a peripheral vein.
“Physiological PK modeling of first-pass drug absorption, metabolism, and excretion in humans—using computationally scaled data from multiple fluidically linked two-channel organ chips—predicts PK parameters for orally administered nicotine (using gut, liver, and kidney chips) and for intravenously injected cisplatin (using coupled bone marrow, liver, and kidney chips),” the authors of the second article wrote. “The quantitative in-vitro-to-in-vivo translation of PK and PD parameters and the prediction of drug absorption, distribution, metabolism, excretion, and toxicity through fluidically coupled organ chips may improve the design of drug-administration regimens for Phase I clinical trials.”
Applying mass spectrometry analysis, the Wyss team quantified nicotine levels in the AV reservoir and the effluents of the vascular channels of all the different organ chips, and then fitted the data with a newly developed biomimetic scaling approach that translates them from the dimensions of the organ chips to their actual organ dimensions in the human body.
For the first time, this computational approach combined with experimental human organ chip data demonstrated the ability to model drug uptake and metabolism, and quantitatively predict dynamic changes in drug blood levels (PK) that previously were observed in human clinical trials. The scaling approach also solves the challenge of drug adsorption into materials in the experimental system.
The two studies answer a challenge issued by the Defense Advanced Research Projects Agency (DARPA), requested grant applications in 2012 with a seemingly impossible challenge: develop 10 types of organ chips that recapitulate the complex functionalities of 10 different human organs, engineer an automated instrument to fluidically link them to create a functional human body chips platform, and leverage computational modeling in combination with experimental data generated using this platform to quantitatively predict human drug PK/PD behavior in vitro.
PK characterization refers to the quantification of a drug’s absorption, distribution, metabolism, and excretion (ADME), which together determine drug levels in the blood. These responses involve interplay between many different organs linked by a vasculature containing flowing blood. PD characterization refers to the quantification of a drug’s effects on its target organs, which underlies its mechanism of action as well as its adverse effects.
PK/PD characterization need not rely on animal models if it can make use of body chips, or linked organ chips. Organ chips are microfluidic culture devices composed of a clear flexible polymer the size of a computer memory stick, which contains two parallel hollow channels that are separated by a porous membrane. Organ-specific cells are cultured on one side of the membrane in one of the channels, and vascular endothelial cells recapitulating a blood vessel line the other, while each channel is independently perfused with cell type-specific medium. The porous membrane allows the two compartments to communicate with each other, and to exchange molecules like cytokines, growth factors, and drugs, as well as drug breakdown products generated by organ-specific metabolic activities.
Because the Wyss Institute’s organ chips contain an endothelium-lined vascular channel, Ingber proposed in 2011 that it might be possible to create a human body chip. Reflecting on the DARPA challenge and the Wyss Institute’s response, Ingber declared, “We were very proud to obtain major funding support from DARPA to take on this challenge, and we are now even more proud that we have successfully met their goal, which would not have been possible without the exceptional talents, interdisciplinary spirit, and monumental team effort at the Wyss Institute.”