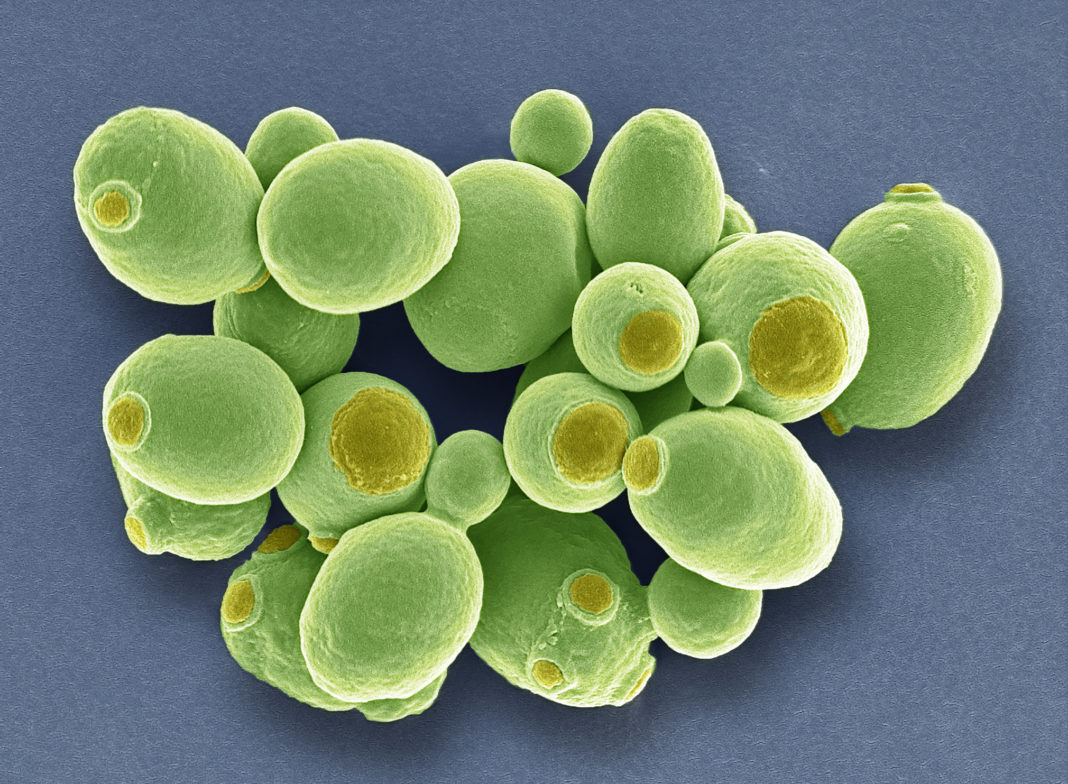
Probiotics—live microorganisms in fermented foods, dietary supplements, and beauty products that have health benefits when consumed or applied to the body—include the yeast, Saccharomyces boulardii. Most species of yeast cannot survive in mammalian guts— the heat and acidity are beyond their limits of tolerance. But S. boulardii not only grows merrily in the mammalian gut, it also inhibits pathogenic gut infections in the mammalian host.
In a new step forward for synthetic biology, researchers report engineering new biosynthetic pathways in S. boulardii enabling the production of vitamin precursors (such as beta-carotene, a precursor of vitamin A) and drugs (such as violacein, a natural drug with anti-bacterial, anti-viral, anti-fungal, and anti-tumor properties) in the guts of mice.
“There are clear advantages to being able to engineer probiotics so that they produce the desired molecules right where they are needed,” said Nathan Crook, corresponding author of the study and an assistant professor of chemical and biomolecular engineering at North Carolina State University. “You’re not just delivering drugs or nutrients; you are effectively manufacturing the drugs or nutrients on-site.”
The paper, “In situ biomanufacturing of small molecules in the mammalian gut by probiotic Saccharomyces boulardii,” that appears in the journal ACS Synthetic Biology reports how the researchers accomplished this feat.
The authors measured the amount and fluctuations in protein expression enabled by genetic regulatory elements such as promoters, terminators, and copy number control elements and demonstrated CRISPR-mediated genome editing with high efficiency (95%) in this strain of yeast.
“We were a little surprised to learn that most of the S. cerevisiae [baker’s yeast] tools worked really well in S. boulardii,” Crook said. “Honestly, we were relieved because, while they are genetically similar, the differences between the two species are what make S. boulardii so interesting, from a therapeutic perspective.” Upon establishing the viability of the toolkit, the researchers tested its functionality by modifying S. boulardii to produce beta-carotene.
“On the one hand, beta-carotene is orange—so we could tell how well we were doing just by looking at the colonies of yeast on a petri dish: they literally changed color,” Crook said. “On a more ambitious level, we knew that beta-carotene is a major provitamin A carotenoid, which means that it can be converted into vitamin A by the body—and we knew that vitamin A deficiency is a major public health problem in many parts of the world. So why not try to develop something that has the potential to be useful?”
The investigators reported that S. boulardii forms stable colonies in germ-free mice for over 30 days, competing for space with other resident microbes in the gut whereas in conventional and antibiotic-treated mice S. boulardii forms colonies for a mere one to two days.
The researchers then tested the modified S. boulardii in the mouse model and found that the yeast cells successfully synthesized beta-carotene in the guts of mice. By comparing the total mass of additional beta-carotene recovered in feces with the beta-carotene present in the initial probiotic dose, the authors estimate that the germ-free mice produced 194 microgram beta-carotene in about 14 days.
This proof-of-concept study inspires more questions on the quantity of beta-carotene absorbed by the mice, the biological relevance of the amounts produced, and most importantly, whether the process can be replicated in humans.
“All of those are questions we’ll have to address in future work,” said Crook. “But we’re excited to see what happens and we’re excited that these tools are now publicly available for use by others in the research community.”