Isolating circulating tumor cells (CTCs) from patient blood is the fundamental principle behind liquid biopsy and numerous microfluidic devices have been developed in recent years to increase the efficiency with which these transformed cells are captured. Now, a team of investigators from the University of Illinois at Chicago (UIC) and Queensland University of Technology of Australia have developed a new device to isolate individual cancer cells from patient blood samples. The device may one day enable rapid, cheap liquid biopsies to help detect cancer and develop targeted treatment plans. Findings from the study were published recently in Microsystems & Nanoengineering through an article titled “Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel.”
“This new microfluidics chip lets us separate cancer cells from whole blood or minimally-diluted blood,” explained senior study investigator Ian Papautsky, PhD, professor of bioengineering in the UIC College of Engineering. “While devices for detecting cancer cells circulating in the blood are becoming available, most are relatively expensive and are out of reach of many research labs or hospitals. Our device is cheap, and doesn’t require much specimen preparation or dilution, making it fast and easy to use.”
The ability to successfully isolate cancer cells is a crucial step in enabling liquid biopsy where cancer could be detected through a simple blood draw. This would eliminate the discomfort and cost of tissue biopsies which use needles or surgical procedures as part of a cancer diagnosis. Liquid biopsy could also be useful in tracking the efficacy of chemotherapy over the course of time, and for detecting cancer in organs difficult to access through traditional biopsy techniques, including the brain and lungs.
Yet, isolating circulating tumor cells from the blood is no easy task, since they are present in extremely small quantities. For many cancers, circulating cells are present at levels close to one per one billion blood cells. “A 7.5-mL tube of blood, which is a typical volume for a blood draw, might have ten cancer cells and 35–40 billion blood cells,” said Papautsky. “So, we are really looking for a needle in a haystack.”
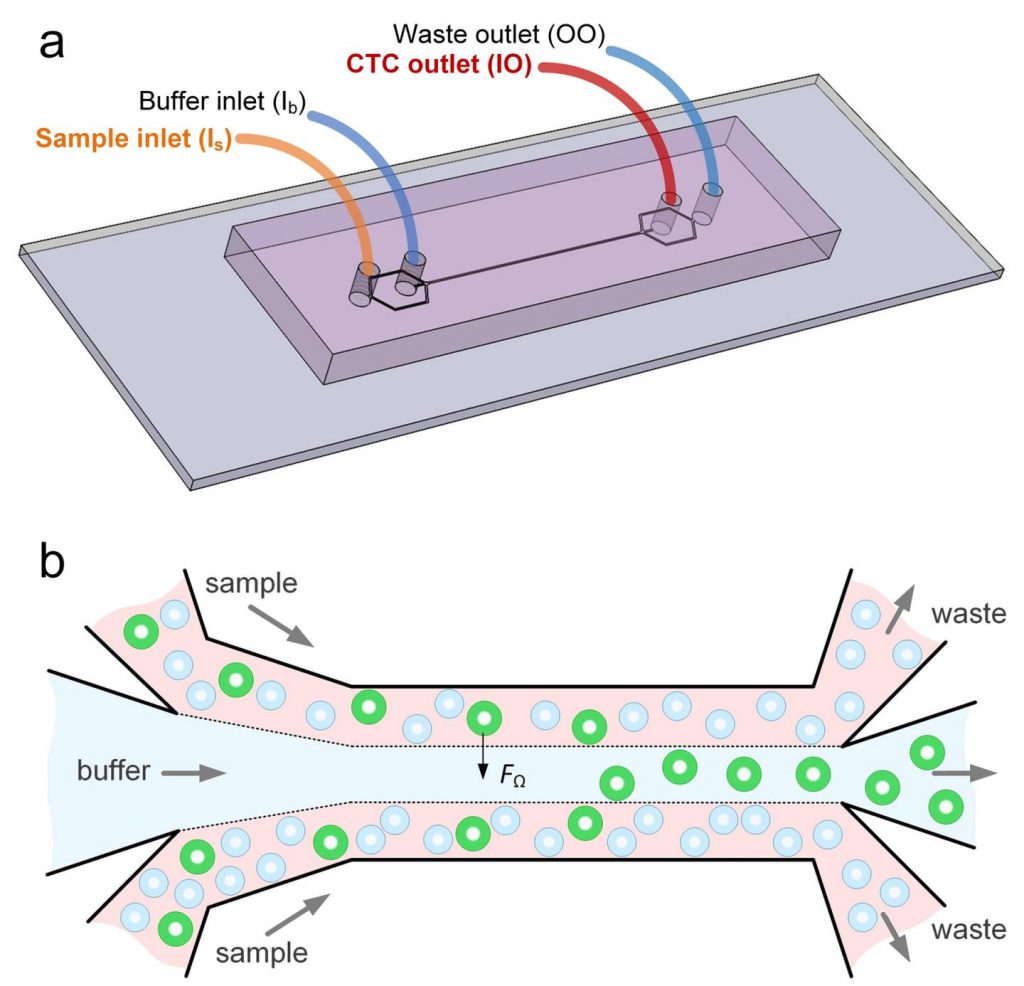
“Using size differences to separate cell types within a fluid is much easier than affinity separation which uses ‘sticky’ tags that capture the right cell type as it goes by,” said Papautsky. “Affinity separation also requires a lot of advanced purification work which size separation techniques don’t need.”
Interestingly, the new microfluidic device capitalizes on the phenomena of inertial migration and shear-induced diffusion to separate cancer cells from blood as it passes through “microchannels” formed in plastic. “We are still investigating the physics behind these phenomena and their interplay in the device, but it separates cells based on tiny differences in size which dictate the cell’s attraction to various locations within a column of liquid as it moves,” Papautsky noted.
Amazingly, when the researchers spiked 5-mL samples of healthy blood with 10 small-cell-lung cancer cells and then ran the blood through their device, they were able to recover 93% of the cancer cells using the microfluidic device. Previously-developed microfluidics devices designed to separate circulating tumor cells from blood had recovery rates between 50–80%.
Additionally, when the team ran eight samples of blood taken from patients diagnosed with non-small-cell lung cancer, they were able to separate cancer cells from six of the samples using the microfluidic device. In addition to the high efficiency and reliability of the devices, the fact that little dilution is needed is another plus.
“Without having to dilute, the time to run samples is shorter and so is preparation time,” Papautsky concluded.




