Researchers headed by a team at the University of Wisconsin (UW)-Madison, and the Morgridge Institute for Research, have developed a novel label-free imaging technique that exploits autofluorescence in cells to differentiate between active and off-duty T cells, at the single cell level. They suggest the technology, known as autofluorescence lifetime imaging, could be used to help evaluate T cell involvement in immunotherapies for cancer treatment or autoimmune diseases. “It’s super novel,” said the Morgridge Institute’s Melissa Skala, PhD, who is also an associate professor of biomedical engineering at UW-Madison. “Most people aren’t using these techniques—you don’t see a lot of autofluorescence studies in immunology.”
Reporting on development and tests with the technology in Nature Biomedical Engineering, the researchers commented, “Autofluorescence lifetime imaging can be used to characterize T cells in vivo in preclinical models, in clinical applications including small blood samples (such as pediatric samples) in which antibody labeling is limited, or in cultured T cells, such as those used in biomanufactured T-cell therapies.” Their paper is titled, “Classification of T-cell activation via autofluorescence lifetime imaging.”
T cells are the immune soldiers at the frontline of the body’s battle with disease-causing pathogens. In a healthy individual, most T cells are in a quiescent, or inactive, state, but remain ready and waiting for the signal to activate and join in the fight against invading virus or bacteria. Most methods for characterizing T cells are antibody-based, such as flow cytometry or immunohistochemistry. These techniques require staining with antibodies or contrast agents, a process that is destructive to the cells. “… these methods require exogenous contrast agents, and flow cytometry and immunohistochemistry require tissue dissociation and fixation, respectively,” the authors wrote. “New tools that are non-destructive and label-free are needed to fully characterize T cells for assessing immunotherapies.”
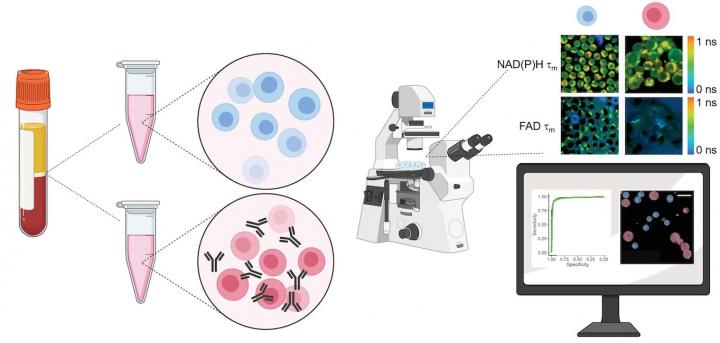
The method developed by Skala and colleagues detects autofluorescence from molecules within the cell that naturally emit light when imaged by a microscope paired with an infrared laser. This label-free process doesn’t damage or alter the behavior of the cell. The technique could be adapted to image cells in a plate or dish, tissue samples, or even in vivo imaging of a complete organism.
“Autofluorescence imaging is an attractive method for analyzing immune-cell behaviors because it is non-destructive, relies on endogenous contrast, and provides high spatial and temporal resolution,” the investigators noted. “T cells have a metabolic switch that regulates their activity,” explained Skala. “We wanted to test if our imaging technology could tell the difference between the quiescent T cells and activated T cells,” added Alexandra Walsh, PhD, formerly an assistant scientist at the Morgridge Institute and who is now an assistant professor of biomedical engineering at Texas A&M University.
To validate their approach, the researchers acquired blood samples from healthy donors, isolated the T cells, and measured autofluorescence of NAD(P)H and FAD, two molecules that are involved in cellular metabolism. “We kept some of the T cells in their quiescent state, and then we added antibodies to a group to activate them,” said Walsh. Images of the quiescent cells in comparison with the activated cells revealed differences in metabolic function, most notably through a change in NAD(P)H autofluorescence in the activated T-cell populations. The team also observed that active T cells were slightly larger in size than quiescent cells.

The authors concluded, “Taken together, autofluorescence lifetime imaging of NAD(P)H and FAD of T cells, combined with machine learning for classification, is an accurate tool for non-destructive label-free assessment of the activation status of T cells … NAD(P)H and FAD autofluorescence lifetime imaging provides high spatial, temporal, and functional information about cell metabolism, which makes it an attractive tool for evaluating T cells.”
The activation protocol and imaging capabilities will be useful for manufacturing the CAR-T cells used in immunotherapies, suggested Skala. These re-engineered T cells are often co-cultured with other cells, such as cancer cells, to test their reactivity. Using additional harsh reagents or antibody labels to further characterize the T cell is a bottleneck for CAR-T cell manufacturers. The autofluorescent approach provides an attractive way to perform those experiments by imaging the same cells across multiple time points in a way that’s non-damaging.
“We showed that you can resolve temporal changes with our imaging technique,” noted Walsh. “We were able to see changes in the imaging endpoints within minutes after adding the activating antibodies.” Walsh further pointed out that it would be difficult to see these dynamic changes using flow cytometry, since the time required for staining and incubation make it difficult to capture multiple timepoints.
The Skala lab plans to continue research to help better understand how a cancer patient’s T cells might respond as the tumor grows or as they’re treated with immunotherapies. “These technologies could tell us something about tumors or about T-cell manufacturing that we didn’t know,” added Skala, “because previously we didn’t have the methods to monitor T-cell behavior over time.”

While this new technique offers many advantages over traditional methods, there are still limitations, the authors acknowledged. For example, autofluorescence imaging isn’t very sensitive. “We aren’t relying on really specific labels, we’re relying on the metabolism of the cells,” Skala said. “That’s only going to get you so far in differentiating the cell types.” Additionally, the technique requires experienced people to perform the microscopic imaging and analyze the data, acknowledged Walsh.
The researchers are also working on developing a prototype to take the imaging capability of their large-scale microscope and translate it into a “box-sized” system. “You won’t have to be a specialized optical engineer to use it,” Skala said. “That’s the direction we’re trying to go. We’re trying to make it more accessible.”