January 1, 2012 (Vol. 32, No. 1)
The Inclusion of Race Elements in Personalize Medicine Patents Ignites Debate
The issue of race in the context of personalized medicine has caught the attention of the press and commentators alike. For example, the Washington Post on July 31, 2011, published an article entitled “Race re-emerges in debate over ‘personalized medicine’.”
The article starts with the remark that examiners at the U.S. Patent and Trademark Office “have rejected patents for genetic screening tests because the applicants did not explore their effectiveness for different races, adding to the debate about whether race has scientific validity in modern DNA-based medicine.” This proposition lends itself to a question relevant to personalized medicine patents, i.e., is the USPTO playing the race card? Although some patents do recite race category elements, I believe the answer is “no”.
Presumably, the Washington Post led with the above-mentioned remark in response to a Nature article published by law professor Jonathan Kahn, entitled “Mandating race: how the USPTO is forcing race into biotech patents.” In his Nature article, Kahn suggests that the USPTO mandates that applicants recite “racial categories” in biotech claims, specifically to overcome enablement rejections by examiners. Kahn describes the dangers of such activity, noting that it promotes the “misleading and potentially dangerous notion that race is a genetic category,” notwithstanding the “complex interaction of race as a social and historical construct with biology.”
In support of his proposition that the “USPTO examiners are requiring applicants to include racial categories,” Kahn refers to his “review of some recent patent cases,” i.e., four patent applications. Notably, of the four, one application is abandoned and two patents include no claims that recite any race or ethnic “category.”
See U.S. Publ. No. 2004/0126794 (application abandoned before responding to a nonfinal Office Action); U.S. Pat. No. 7,579,147 (containing claims that do not recite race or ethnicity); and U.S. Pat. No. 7,838,220 (containing claims that do not recite race or ethnicity). Kahn discusses one case at length, i.e., U.S. Pat. No. 6,716,581 (U.S. Publ. No. 2002/0039733), which includes claims reciting a method of determining relative age-related risk of colorectal cancer in a Hispanic subject.
Regarding the ’147 and ’220 patents, the two referenced patents with claims that do not recite race or ethnicity elements, Kahn asserts that during prosecution, the examiner rejected claims in both cases for lack of enablement because claims too broadly encompassed all racial populations.
Only on appeal did the USPTO Board of Patent Appeals and Interferences (BPAI) reverse such findings and ultimately allow relevant claims as issued. Based on these cases, Kahn states: “One must ask how many applicants simply accede to the examiners’ demands and incorporate racial categories into their patents to avoid the long, drawn-out process of appeal?”
Patent Search
In what appears to be a response to Kahn’s article by the USPTO, the July 31 Washington Post article reports that USPTO officials state that “they do not require applicants to delineate how well any genetic tests—including those looking for an increased risk for diseases—work by race. A search of more than 3 million patents over the past 20 years found only 37 that included any reference to race.”
One USPTO spokesperson further stated that “[w]e recognize that race is a fuzzy concept,” also noting that “[i]t’s the genetic marker that’s important, not the racial group.”
It is noteworthy that the USPTO, at least as a matter of policy, has this viewpoint. As I see it, an important consideration in personalized medicine is an individual’s specific genetic profile, but not necessarily one’s race or ethnicity. In fact, personalized medicine runs the risk of missing its mark altogether if it relies only on race, a category that is dubious when it comes to generalizing as to specific biomarkers and actual genetic profiles.
In this sense, I share Kahn’s concern if it is indeed accurate that USPTO examiners (rightly or wrongly in light of USPTO policy) are “requiring applicants to include racial categories.” Especially if examiners assert that personalized medicine claims must recite racial categories in order to be enabled, such assertions have the danger of being inaccurate scientifically and legally, among other things.
As noted by Kahn, a designation of race involves numerous social and cultural aspects, in addition to subjective considerations of how one sees themselves. Such aspects often have little bearing on whether a particular group has a specific genetic profile, and certainly cannot speak to the genetic profile of every individual in the group. To the extent that a claim reciting a specific genetic profile (e.g., X polymorphism in gene Y) lacks enablement, it does not help the situation to recite a race element.
Even if a rare examiner suggests (incorrectly, as concluded by the BPAI in at least two relevant cases) that a patent claim requires a race category element in order to be enabled, this does not mean “the USPTO is forcing race into biotech patents,” as asserted by Kahn. As the Washington Post article points out, the USPTO recognizes that “[i]t’s the genetic marker that’s important, not the racial group.” Absent evidence that the USPTO is mandating (and training examiners to implement) policies consistent with Kahn’s hypothesis, a random rogue examiner does not mean the sky is falling.
This all said, Kahn’s article prompted me to conduct my own review of relevant claims allowed by the USPTO. I wanted to see for myself whether, as Kahn asserts, “it is evident that the practice of requiring patent applications to introduce race into their biotech patents has become routinized at the USPTO.”
A USPTO official stated to the Washington Post that “[a] search of more than 3 million patents over the past 20 years found only 37 that included any reference to race.” Even assuming examiners have, in fact,“forced” race elements into claims in every one of the 37 cases (an unlikely scenario), 37 patents fails to indicate a “routine” situation, at least to me.
My own preliminary search on the USPTO website revealed more than 37 patents reciting a race element, but not many more. For example, I conducted a search on the USPTO website for issued patents having claims reciting “African American” or “Asian” or “Hispanic” or “Caucasian.” This search received 128 “hits.” A quick review of these hits indicated that many patents were related to plants, insects, or subject matter otherwise irrelevant to personalized medicine.
Looking at the first 50 hits of this search, I uncovered 19 relevant U.S. patents, which are outlined in the Table. A review of the other 78 (of 128) hits uncovered 16 additional relevant patents (including U.S. Pat. No. 6,716,518 discussed in Kahn’s article).
In other words, this single search uncovered 35 relevant patents. I also conducted a few other preliminary searches using terms such as (Jewish or Filipino), (descen$ or ancestry), (treat$ or diagnos$ or screen$) and/or (human or patient or “biological sample”). Relatively narrow searches using such terms uncovered five additional patents, bringing my total to 40.
Before relying on this number, however, I should note that I conducted additional broader searches, but hit numbers were large (>500), with many hits being irrelevant. For example, adding the term “Japanese” to my initial search (i.e., “African American” or “Asian” or “Hispanic” or “Caucasian” or “Japanese”) resulted in 1161 hits, a significant jump from 128. Substituting “European” for “Japanese” in the search resulted in 727 hits. Substituting terms such as “Black” or “White” resulted in hits greater than 20,000. I did not review these larger search results. Thus, my searches discussed herein are not conclusive of the total number of U.S. issued patents containing claims that reference race.
Looking at the Table, however, a few things pop out. First, the USPTO has issued, and continues to issue, personalized medicine patents with claims that recite race elements. Second, in most such cases, independent claims recite the race element in question, not merely a dependent claim. Third, the claims recite specific genetic parameters in addition to the race element, which may render the race element irrelevant to patentability (including enablement).
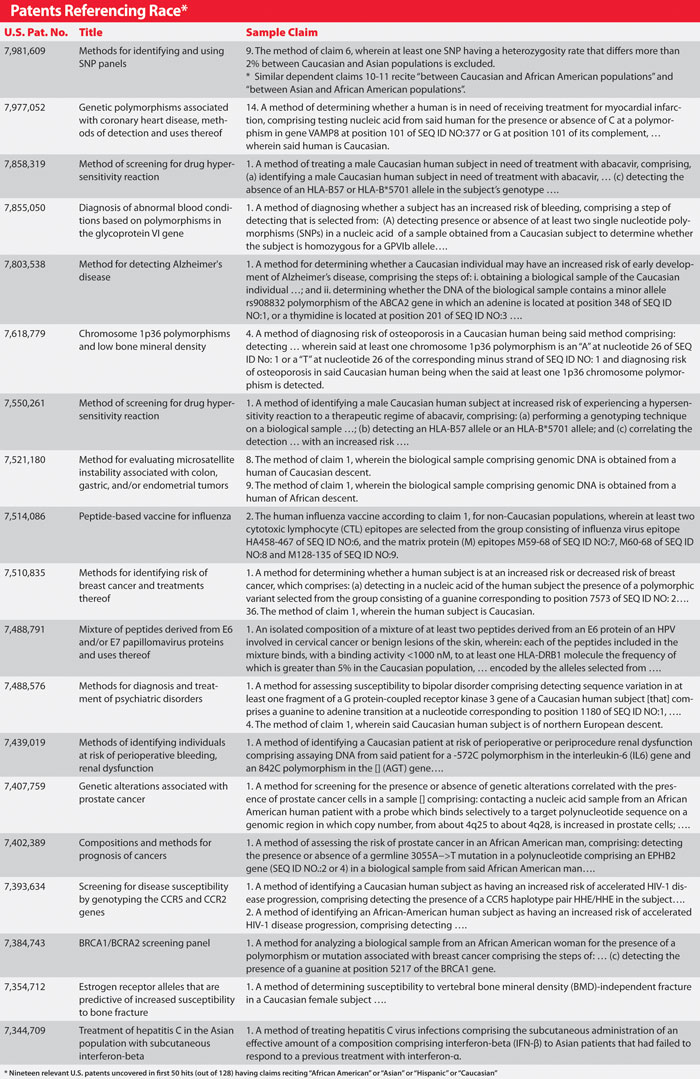
Relevant Patents
Finally, it appears to be the case, as the USPTO suggests, that the total number of relevant patents is quite low especially compared to the number of issued patents relating to personalized medicine and diagnostics in general. In addition, even among relevant patents discussed here (notwithstanding Kahn’s discussion of U.S. Pat. No. 6,716,518), it is possible that applicants insert race language on their own, without any prompting by the USPTO, much less via an examiner “forcing” them to do so.
In his Nature article, Kahn concludes that “[t]he truly chilling aspect of this story are the questions it raises as to how many other applicants have given in to examiner’s demands that they include race in their patents?” Likewise, the Washington Post article concludes with Kahn’s statement that “[t]here’s no telling how many people will just give in and use race in a way that the scientists clearly do not think is an appropriate way to use race.”
If the USPTO had a policy to require personalized medicine patent claims to specify race, I might share these concerns, but the few patents identified in my searches do not suggest a widespread practice. It is a fact of patent practice that examiners often ask applicants to make their patent claims more narrow, and it is up to applicants to stand their ground when suggested amendments do not make sense scientifically or legally.
If an examiner tries to play a race card, the applicant should call his bluff, and perhaps offer to include more scientifically relevant claim language to address the examiner’s concerns.
Courtenay C. Brinckerhoff ([email protected]) is a partner, member, and vice-chair of the chemical, biotechnology & pharmaceutical practice of Foley & Lardner, and editor of PharmaPatentsBlog.com.


