Scientists at the Liverpool School of Tropical Medicine (LSTM) and Imperial College London have designed a new type of drug against influenza that targets the flu virus’ ability to gain entry into its host’s cells. The new technology, reported in the Journal of Immunology, effectively engineers extra sialic acid into antibody fragments, which can then bind tightly to the viral haemagglutinin (HA) and neuraminidase (NA) proteins that the pathogen uses to infect human cells. The researchers suggest their approach could lead to the development of more effective flu vaccines, but potentially could also be applied to develop drugs against other diseases. The platform is available for licensing, and is already attracting potential commercial interest, GEN was told by LSTM research lead Richard Pleass, PhD.
“The crystal structure of influenza HA published in 1981 triggered a search for small molecules inhibitors with high-affinity binding to HA,” he explained to GEN. “Despite 38 years of searching no competitive inhibitor with therapeutic potential was found. This is due to the weak interaction of HA to its natural ligand sialic acid.” The researchers reported on how they have overcome this issue and strengthened IgG antibody Fc fragment binding to these key proteins. Their paper is titled, “Insertion of N-Terminal Hinge Glycosylation Enhances Interactions of the Fc Region of Human IgG1 Monomers with Glycan-Dependent Receptors and Blocks Hemagglutination by the Influenza Virus.”
Flu is responsible for some 300,000–650,000 respiratory deaths globally every year, primarily in children and the elderly. Last year represented the 100th anniversary of the 1918 influenza pandemic that killed nearly 100 million people around the world. The ability of the flu virus to mutate still thwarts efforts to develop a universal flu vaccine that could target multiple strains and be effective in the event of another pandemic.
“Influenza vaccines have limited public health impact during pandemics, and current influenza vaccines are less efficacious than vaccines for many other infectious diseases,” Pleass noted in a statement. “This is because influenza viruses that circulate in human and animal populations mutate two key viral surface proteins, HA and NA, thus allowing them to escape from protective antibodies produced through natural infection or vaccination.”
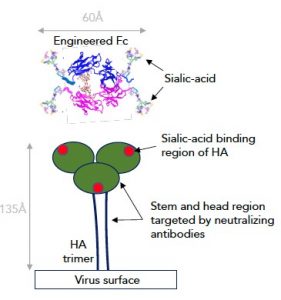
Both HA and NA bind the sialic acid sugar that is found on the surface receptors of cells in the mammalian airway that the flu virus infects. These sialic acid-targeting regions of the HA and NA proteins need to stay conserved so that they can bind sialic acid. Capitalizing on this feature, the U.K.-based researchers have succeeded in engineering IgG antibody Fc fragments that are enriched for sialic acid, and which effectively target and bind HA and NA, blocking their interaction with the human cells. Importantly, the modified Fc fragments should be suitable both as clinical candidates, and for large-scale manufacture.
“The strong interaction between virus and host cell is caused by multivalent binding of trimeric HA to numerous carbohydrate moieties on the surface of the cell,” Pleass further explained to GEN. “Although sialylated multimeric scaffolds e.g., nanoparticles have been shown to exert tight binding, current regulatory/licensing systems are not up to the challenges of delivering these novel scaffolds to humans safely … In contrast, Fc fragments are a well understood class of compound and have even been used to treat idiopathic thrombocytopenia (ITP) in children. They are also straightforward to manufacture using existing antibody pipelines available for CHO [chinese hamster ovary] cells, the manufacturing workhorse of the pharmaceutical industry.”
The new technology could provide an answer to the urgent need for better anti-influenza drugs. As Pleass commented, “The transfer of antibodies from people recovering from influenza during the 1918 and 2009 pandemics reduced mortality from influenza by 50% and 26% respectively. However, to be useful, these antibody medicines (also called FLU-IVIG) need to be manufactured in advance of future epidemics, which is obviously problematic as there may be modest or little neutralizing activity against newly emerging strains. Therefore, combinations of existing medicines, including FLU-IVIG, with sialic acid blockers could increase their efficacy while future-proofing against the next pandemic.”
The new sialic acid-modified Fc molecules are 75% sialylated, compared with just 2% for an IgG-Fc control, the researchers reported in their published paper. “… we were able to identify monomeric Fc glycan mutants with enhanced binding to influenza A virus hemagglutinin that inhibited viral-mediated agglutination of human erythrocytes.” And as influenza isn’t the only pathogen to use glycans as an infection route, the sialic acid-modified Fc technology could be developed to target a range of different diseases. … As many pathogens rely on glycans to infect host cells, these reagents may also be useful as inhibitors of infection,” the authors wrote.
“… by adding or removing glycosylation and/or disulphide-bonding sites within our original hexameric Fc platform, new repertoires of desirable binding attributes can be made. These molecules may be useful in the control of other pathogens, including Newcastle disease virus, group B streptococci, Streptococcus pneumoniae, and Mycoplasma genitalium, in which sialic acid–dependent interactions are also crucially important.”
“This is a fascinating project, and one which could have really far-reaching impact not only for influenza but as a platform technology to develop new medicines for many other diseases that are currently treated by antibodies,” stated Sara Marshall, PhD, head of clinical and physiological sciences at the Wellcome Trust, which provided funding for this work.