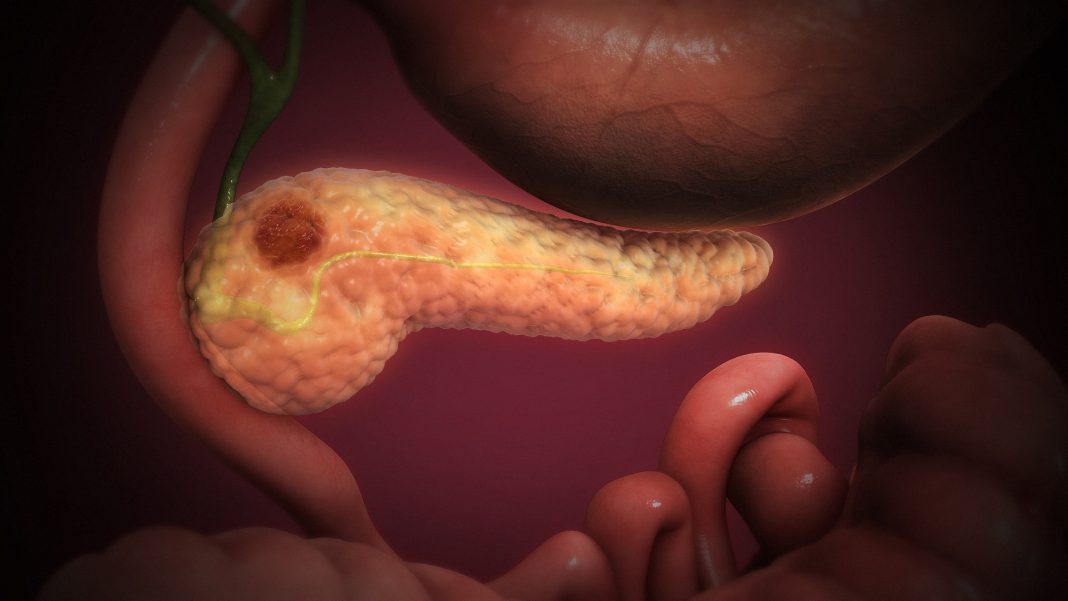
Hunkered behind a dense layer of fibrotic tissue, pancreatic tumors stubbornly resist the delivery of therapeutic molecules. Yet this barrier, this desmoplastic tumor tissue, can be penetrated by iRGD, a nine-amino-acid cyclic peptide. In experiments with models of pancreatic cancer, specifically, mouse models and organoids, iRGD was found to penetrate tumors via a molecular pathway mediated by β5 integrin, a protein that is produced by carcinoma-associated fibroblasts (CAFs). This newfound role for β5 integrin suggests that the protein may serve as a useful biomarker, one that could indicate which patients will respond best to therapies that combine iRGD and chemotherapeutic agents.
The new findings come from a study conducted by researchers at the University of California, San Diego (UCSD), School of Medicine and Moores Cancer Center, in collaboration with researchers at Sanford-Burnham-Prebys Medical Discovery Institute and Columbia University. “The knowledge gained from our study has the potential to be directly applied to patient care,” said Andrew Lowy, MD, chief of the division of surgical oncology at Moores Cancer Center. “We also believe that the levels of β5 integrin within a pancreatic cancer could tell us which patients would benefit the most from iRGD-combination therapy.”
Lowry, who is also professor of surgery at UCSD School of Medicine, is the co-corresponding author of a paper about the new study. This paper (“Tumor-penetrating therapy for β5 integrin-rich pancreas cancer”), which appeared March 9 in Nature Communications, reported that CAFs induce β5 integrin expression in tumor cells in a TGF-β dependent manner, making them an efficient drug delivery target for the tumor-penetrating peptide iRGD.
“The capacity of iRGD to deliver conjugated and co-injected payloads is markedly suppressed when β5 integrins are knocked out in the tumor cells,” the article’s authors wrote. “Of note, β5 integrin knock-out in tumor cells leads to reduced disease burden and prolonged survival of the mice, demonstrating its contribution to PDAC progression.”
PCAD, or pancreatic ductal adenocarcinoma, is a subtype of pancreatic cancer that is highly drug-resistant due, in part, by the hard shell-like outer layer surrounding the tumor. Every 12 minutes, someone in the United States dies of pancreatic cancer, which is often diagnosed late. It spreads rapidly and has a five-year survival rate of approximately 10%. Treatment may involve radiation, surgery, and chemotherapy, though often the cancer becomes resistant to drugs.
According to the new study, the tumor-penetrating peptide known as iRGD appears to enhance the effects of chemotherapy, reducing metastasis, and increasing survival, to a degree determined by levels of β5 integrin. Essentially, β5 integrin gives chemotherapeutic agents the opportunity to destroy the tumor from within.
“This type of tumor is made up of a dense fibrous tissue that acts as a barrier to drugs trying to get through,” explained Tatiana Hurtado de Mendoza, PhD, first author of the new study and assistant project scientist at UCSD School of Medicine and Moores Cancer Center. “Many drugs can reach the vessels of the tumor, but they are not able to get deep into the tissue, making treatment less effective, and that is one reason why this type of cancer is so challenging to treat.
“Our study found that the tumor-penetrating peptide iRGD is able to use this fibrous network to deliver chemotherapy drugs deep into the tumor and be more effective.”
The research team examined the microenvironment of PDAC tumors in a mouse model. They found that after targeting the tumor blood vessels, iRGD binds to high levels of β5 integrin, a protein produced by CAFs, cells that produce much of the tumor’s protective fibrous cover.
“We were able to closely replicate human disease in our mouse model and found that when iRGD was injected with chemotherapy in mice with high levels of β5 integrin, there was a significant increase in survival and a reduction in the cancer spreading to other organs in the body compared to chemotherapy alone,” said Lowy. “This could be a powerful treatment strategy to target aggressive pancreatic cancer.
“What is also exciting about this finding is the iRGD therapy did not produce any additional side effects. This is critically important when considering treatments for patients.”
The researchers said next steps include a national human clinical trial. They estimate the trial could begin in one year. “As clinical investigation of iRGD advances to its next stages,” the authors of the Nature Communications article concluded, “patient stratification based on the expression profiles of iRGD-binding proteins as putative biomarkers, β5 integrin in particular, will be of great interest and importance.”