Aging well can mean different things to different people. But minimizing the effects that aging has on health is something most can agree on. Why? For one, elderly people are more prone to infectious diseases as the function of their immune system continuously declines with progression of age. In addition to the age-related immune decline, aged individuals are commonly affected by frailty that negatively impacts quality-of-life. Even though the average life-expectancy for humans continues to rise, living longer is often associated with age-related health issues.
Now, a group of researchers from Switzerland have identified new approaches to improve health-span in a fast-growing aging population. For many years, scientists speculated that chronic low-grade inflammation accelerates aging processes and the development of age-related disorders. An international team of researchers has now demonstrated that visceral adipose tissue, known as belly fat, crucially contributes to the development of chronic low-grade inflammation.
Their work is published in the paper, “Eosinophils regulate adipose tissue inflammation and sustain physical and immunological fitness in old age,” in Nature Metabolism.
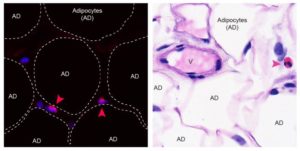
The authors noted that adipose tissue eosinophils (ATEs) are important in the control of obesity-associated inflammation and metabolic disease. However, the way in which aging impacts the regulatory role of ATEs remains unknown. In this work, the team shows that certain immune cells play an essential role in regulating chronic low-grade inflammation and downstream aging processes. And, that restocking fat tissues of old mice with eosinophils prevents age-related declines in physical and immunological functions. They could show, that these immune cells may be used to reverse such processes.

Belly fat as a source of chronic inflammation
The team demonstrated eosinophils, which are predominantly found in the blood, are also present in belly fat of both humans and mice. Although classically known to provide protection from parasite infection and to promote allergic airway disease, eosinophils located in belly fat are responsible for maintaining local immune homeostasis. With increasing age the frequency of eosinophils in belly fat declines, while the number of pro-inflammatory macrophages increases. Owing to this immune cell dysbalance, belly fat turns into a source of pro-inflammatory mediators accumulating systemically in old age.
In a next step, the researchers investigated the possibility to reverse age-related impairments by restoring the immune cell balance in visceral adipose tissue.

“In different experimental approaches, we were able to show that transfers of eosinophils from young mice into aged recipients resolved not only local but also systemic low-grade inflammation,” said Alexander Eggel, PhD, independent research group leader, University of Bern, Switzerland.
The authors wrote that “ATEs undergo major age-related changes in distribution and function associated with impaired adipose tissue homeostasis and systemic low-grade inflammation in both humans and mice.” Further, they found that “exposure to a young systemic environment partially restores ATE distribution in aged parabionts and reduces adipose tissue inflammation.”
“In these experiments, we observed that transferred eosinophils were selectively homing into adipose tissue,” added Mario Noti, PhD, senior scientist immunology, Nestlé Research, Lausanne, Switzerland.
“Approaches to restore ATE distribution using adoptive transfer of eosinophils from young mice into aged recipients proved sufficient to dampen age-related local and systemic low-grade inflammation,” noted the authors. Importantly, they added, “restoration of a youthful systemic milieu by means of eosinophil transfers resulted in systemic rejuvenation of the aged host, manifesting in improved physical and immune fitness that was partially mediated by eosinophil-derived IL-4.”
Translating findings into clinics
“Our results indicate that the biological processes of aging and the associated functional impairments are more plastic than previously assumed,” stated Noti. Importantly, the observed age-related changes in adipose immune cell distribution in mice were also confirmed in humans. “A future direction of our research will be to now leverage the gained knowledge for the establishment of targeted therapeutic approaches to promote and sustain healthy aging in humans,” said Eggel.



