Huntington’s disease (HD) is a neurodegenerative disorder in which the clinical symptoms appear in adulthood. Despite the lack of symptoms earlier in life, the mechanism by which aging drives the onset of neurodegeneration in patients with HD remains unclear. Now, scientists have studied striatal medium spiny neurons (MSNs) directly reprogrammed from fibroblasts of patients with HD. Huntington’s disease specifically destroys the medium spiny neurons, the loss of which causes involuntary muscle movements, impaired mental health, and cognitive decline. Patients typically live about 20 years after signs of the disease first appear.
By modeling the age-dependent onset of pathology, the researchers found that, as patients age, the disease gradually impairs autophagy—a process responsible for eliminating waste from cells. This housekeeping is significant in Huntington’s because a buildup of waste leads to cell death. They went on to show that enhancing the autophagy pathway in such neurons that were created from skin cells of Huntington’s patients protects those cells from dying.
This work appears in Nature Neuroscience in the paper, “Age-related Huntington’s disease progression modeled in directly reprogrammed patient-derived striatal neurons highlights impaired autophagy.”
“Our study reveals how aging triggers a loss of the crucial process of autophagy—and hints at how we might try to restore this important function, with the aim of delaying or even preventing Huntington’s disease,” said Andrew Yoo, PhD, a professor of developmental biology at the Washington University School of Medicine in St. Louis.
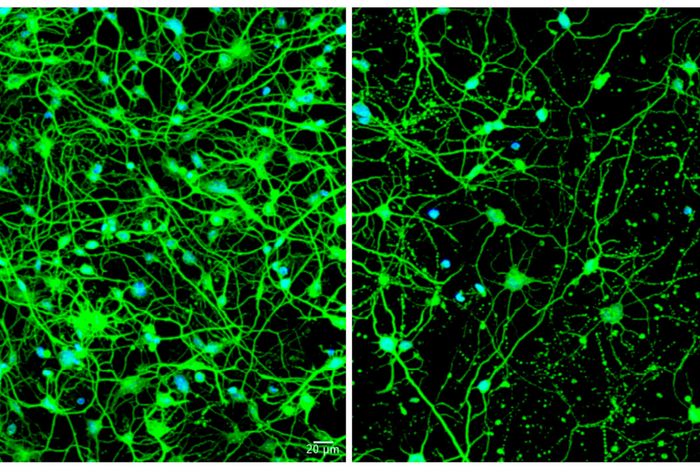
For this study, the researchers reprogrammed patients’ skin cells into medium spiny neurons. More common techniques involve use of stem cells—but stem cells reset the cells’ biological clocks to an early developmental state, which is not useful when studying diseases that only become symptomatic in adulthood.
“We collected skin cell samples from different patients at a range of ages and modeled the disease before and after symptoms developed, which allowed us to identify the differences between younger and older patients with Huntington’s disease,” Yoo said. “We knew there must be some change that takes place as patients age. They all have a genetic mutation in the Huntingtin gene. We wanted to find the difference between young patients who have no symptoms and older patients who actively show signs of the disease.”
The team found that medium spiny neurons reprogrammed from skin cells of older patients with symptomatic Huntington’s produced very high levels of the microRNA, miR-29b-3p. These high levels were not seen in reprogrammed neurons of younger Huntington’s patients or in reprogrammed neurons from healthy individuals of any age. The investigators showed that the microRNA set off a chain of events that included impairing autophagy in these cells. When the skin cells completed the conversion into neurons, they began producing the problematic microRNA, autophagy slowed down, and the cells began dying.
The researchers went on to show that reducing levels of this microRNA allowed autophagy to continue and protected the neurons from dying. In addition, they found that enhancing autophagy with a chemical compound called G2 protected the diseased neurons from death. As the researchers increased the dose of G2, the protection from cell death improved as well.
G2 was identified via high throughput screening for autophagy enhancer drugs that could correct the cellular accumulation of variant alpha-1-antitrypsin Z that causes liver disease in alph-1-antitrypsin deficiency (ATD). The G2 compounds could therefore represent attractive candidates for preventing neurodegeneration in Huntington’s disease, liver disease in alpha-1-antitrypsin deficiency, and perhaps other diseases in which aberrant accumulation of misfolded proteins is toxic to cells.
The study also uncovered what may be a tantalizing clue for understanding cognitive decline in normal aging. When comparing the symptomatic neurons to pre-symptomatic neurons and to healthy neurons from both young and older adults, the researchers found that the neurons of healthy older adults produced slightly elevated levels of the harmful microRNA, but in far smaller amounts than the neurons of symptomatic Huntington’s disease patients. The study suggests that even in normal, healthy aging, medium spiny neurons gradually produce low levels of this microRNA, which may interfere with autophagy’s healthy cellular housekeeping.
“By modeling different stages of the disease across the life span, we can identify how aging plays a role in disease onset,” Yoo said. “With that information, we can begin to look for ways to delay that onset. Our study also suggests that the triggering molecule for the onset of Huntington’s disease may play some role in age-associated decline in neuronal function generally. Understanding the component of aging that sets off neurodegeneration may help in developing new strategies for treatment and prevention of Huntington’s disease and other neurodegenerative conditions that develop at older ages.”
Yoo and his team are also working with other collaborators using their cellular reprogramming technique to investigate forms of Alzheimer’s disease, tauopathy, and other neurodegenerative conditions.