High drug prices are not justified by industry’s spending on R&D, according to an article “High drug prices are not justified by industry’s spending on research and development” published in The BMJ.
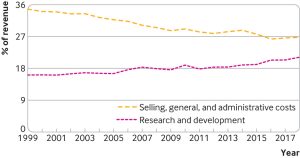
Aris Anelis, PhD, assistant professor in health economics with the department of health services research and policy at the London School of Hygiene and Tropical Medicine (LSHTM), and colleagues pointed out that from 1999 to 2018, the world’s 15 largest biopharmaceutical companies spent more on selling, general, and administrative activities (which includes marketing) than on R&D and that most new medicines developed during this period offered little or no clinical benefit over existing treatments.
By refocusing their spending, they say drug companies “could provide more innovative drugs at affordable prices,” and they call for government action to encourage R&D oriented to public health priorities.
Concerns over the prices of new medicines have been growing over the past decade, they wrote. In the United States, estimated net prices of newly launched prescription drugs increased from an average of around $1,400 a year (£1,200; €1,300) in 2008 to over $150,000 a year in 2021, and even old and common drugs have seen inexplicable price increases in recent years.
Questions have been raised
The biopharmaceutical industry has long argued that high prices are needed to sustain R&D for new medicines. And while the authors acknowledged that there are large financial risks associated with bringing new medicines to market, they say analysis of drug company spending relative to products raises questions about this claim.
For example, publicly available financial reports from 1999 to 2018 show that the 15 largest biopharmaceutical companies had total revenues of $7.7tr. Over this period, they spent $2.2tr on costs related to selling, general, and administrative activities and $1.4tr on R&D.
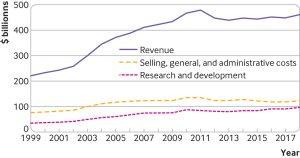
Most of the same companies also spent more buying their own stocks (share buybacks) than on R&D during this period, noted the authors.
The justification of high drug prices to offset R&D spending also ignores the sizeable public investments in drug discovery and development, they added. This means that society is potentially paying twice for new drugs, first in the form of publicly subsidized research and second through high product prices, according to the authors.
Clinical benefits
What’s more, most new drugs provide little or no added clinical value, they explained. For instance, in the 1970s and the 1980s, around 1 in 6 (16%) new drugs approved by the FDA offered important therapeutic gains. Yet analyses of drug evaluation reports by health technology assessment bodies in France and Germany in the 2010s suggest that most new drugs offer little or no added clinical value, with only a fraction offering important or major improvements.
Angelis and colleagues acknowledged that, on the positive side, most products under development during 1997–2016 targeted novel mechanisms of action, but say there has also been a shift in focus from blockbuster drugs, typically targeting chronic diseases and sold in high volumes globally, to “nichebuster” drugs targeting rare diseases or narrow indications for which high prices can be charged.
“Given the amount spent on non-research and development activities and that most new drugs add little or no therapeutic value, in theory, the biopharmaceutical industry could generate more medically valuable innovation with its existing resources,” they say. “This is unlikely to happen, however, without government intervention or regulation along the lifecycle of new medicines.”
As such, they argue that governments, policymakers, drug regulators, health technology assessment bodies, and payers “need to re-think the incentives for valuable biopharmaceutical innovation, creating policy and regulatory environments that will meet public health objectives.
“The world needs a truly value-based health-industrial ecosystem for incentivizing and rewarding improvements in health outcomes and population health throughout the lifecycle of new medicines,” they concluded.
GEN contacted two industry trade groups, the Biotechnology Innovation Organization (BIO) and the Pharmaceutical Research and Manufacturers of America (PhRMA), for comments on the study, but did not receive a reply from either organization.


