Scientists at the University of Southern California Alfred E. Mann Department of Biomedical Engineering say they have developed a “heart attack on a chip,” a device that could one day serve as a method for developing new heart drugs and even personalized medicines.
“Our device replicates some key features of a heart attack in a relatively simple and easy-to-use system,” said Megan McCain, PhD, an associate professor of biomedical engineering and stem cell biology and regenerative medicine, who developed the device with postdoctoral researcher Megan Rexius-Hall, PhD.
“This enables us to more clearly understand how the heart is changing after a heart attack. From there, we and others can develop and test drugs that will be most effective for limiting the further degradation of heart tissue that can occur after a heart attack.”
McCain, a cardiac tissue engineer, whose work previously included co-developing a heart on a chip, and Rexius-Hall detail their findings in an article (“A Myocardial Infarct Border-Zone-On-A-Chip Demonstrates Distinct Regulation of Cardiac Tissue Function by an Oxygen Gradient”) in Science Advances.
“After a myocardial infarction, the boundary between the injured, hypoxic tissue and the adjacent viable, normoxic tissue, known as the border zone, is characterized by an oxygen gradient. Yet, the impact of an oxygen gradient on cardiac tissue function is poorly understood, largely due to limitations of existing experimental models,” the investigators wrote.
A microphysiological system
“Here, we engineered a microphysiological system to controllably expose engineered cardiac tissue to an oxygen gradient that mimics the border zone and measured the effects of the gradient on electromechanical function and the transcriptome. The gradient delayed calcium release, reuptake, and propagation; decreased diastolic and peak systolic stress; and increased expression of inflammatory cascades that are hallmarks of myocardial infarction. These changes were distinct from those observed in tissues exposed to uniform normoxia or hypoxia, demonstrating distinct regulation of cardiac tissue phenotypes by an oxygen gradient.
“Our border-zone-on-a-chip model advances functional and mechanistic insight into oxygen-dependent cardiac tissue pathophysiology.”
However, scientists don’t completely understand this process, especially how heart cells in the healthy and injured parts of the heart communicate with each other and how and why they change after a heart attack.
McCain and Rexius-Hall believe their heart attack on a chip can shed some light on those mysteries. “Fundamentally, we want to have a model that can lead to a better understanding of heart attack injury,” Rexius-Hall said.
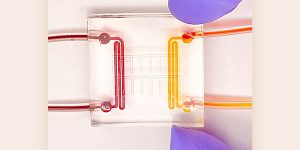
Built from the ground up
The heart attack on a chip is literally built from the ground up. At the base is a 22-mm-by-22-mm square microfluidic device made from a rubber-like polymer called PDMS (with two channels on opposing sides through which gases flow).
Above that sits a thin layer of the same rubber material, which is permeable to oxygen. A micro layer of protein is then patterned on the top of the chip, “so that the heart cells align and form the same architecture that we have in our hearts,” McCain said. Finally, rodent heart cells are grown atop the protein.
To mimic a heart attack, gas with oxygen and gas without oxygen is released through each channel of the microfluidic device, “exposing our heart on a chip to an oxygen gradient, similar to what really happens in a heart attack,” McCain explained.
Because the microfluidic device is small, clear, and easy to see on a microscope, it also allows researchers to observe in real-time the functional changes that sometimes happen in the heart after an attack, including an arrythmia, or an irregular heartbeat, and contractile dysfunction, or decreases in the contraction strength of the heart.
In the future, researchers may make the model more complex by adding immune cells or fibroblasts, the cells that generate the scar after a heart attack.
By contrast, researchers cannot watch changes to heart tissue in real-time with animal models. Additionally, traditional cell culture models uniformly expose heart cells to high, medium, or low levels of oxygen, but not a gradient. That means they cannot mimic what really happens to damaged heart cells in the so-called border zone after a heart attack, pointed out Rexius-Hall.


