Scientists at Albert Einstein College of Medicine have developed a new anticancer strategy that they suggest could bolster the effectiveness of immune-checkpoint therapy. The team said their findings also point to a mechanism that may explain why the latest anti-TIGIT treatments may be failing in the clinic.
Rather than rally T cells against cancer, the Einstein research team focused on natural killer (NK) cells, and developed a monoclonal antibody to block a protein, KIR2DL5, on NK cells, which binds to a protein called PVR that is overexpressed in cancer. The researchers’ studies in mouse models of human cancers confirmed that blocking KIR2DL5 reduced tumor growth and improved survival.
“We believe the novel immunotherapy we’ve developed has great potential to move into clinical trials involving various types of cancer,” said study lead Xingxing Zang, MMed, PhD, the Louis Goldstein Swan chair in cancer research and professor of microbiology & immunology, of oncology, of urology, and of medicine at Einstein and a member of the Cancer Therapeutics Program of the Montefiore Einstein Cancer Center.
Zang and colleagues reported on their developments in the Journal of Clinical Investigation, in a paper titled, “Blockade of the immunosuppressive KIR2DL5-PVR pathway elicits potent human NK cell-mediated anti-tumor immunity.” In their paper the researchers noted, “Blockade of KIR2DL5 with our new blocking mAb significantly enhanced NK-mediated anti-tumor immunity both in vitro and in vivo, demonstrating blockade of the KIR2DL5-PVR pathway as a new immunotherapy for treating human cancers.”
The surfaces of immune cells are studded with receptors known as checkpoint proteins, which prevent immune cells from straying beyond their usual targets of pathogen-infected cells and cancer cells. When checkpoint receptors on immune cells bind with proteins expressed by the body’s own normal cells, the interaction puts the brakes on a possible immune cell attack. Most types of cancer cells also express proteins that bind with checkpoint proteins, tricking immune cells into standing down and not attacking the tumor.
Immune checkpoint inhibitors are monoclonal antibodies designed to short-circuit immune cell/cancer cell interactions by blocking either the tumor proteins or the immune cell receptors that bind with the tumor proteins. With no brakes to impede their activity, immune cells can then attack and destroy cancer cells.
Immune checkpoint inhibitors work by unleashing the immune system’s T cells to attack tumor cells. Their introduction a decade ago marked a major advance in cancer therapy, but only 10–30% of treated patients experience long-term improvement.
The limited effectiveness of checkpoint inhibitors prompted Zang and other scientists to look at checkpoint pathways involving NK cells, which, like T cells, play major roles in eliminating unwanted cells. A cancer-cell protein called PVR was of particular interest.
PVR protein is usually absent or very scarce in normal tissues but is found in abundance in many types of tumors including colorectal, ovarian, lung, esophageal, head and neck, stomach, and pancreatic cancer as well as myeloid leukemia and melanoma. “Accumulating evidence suggests that PVR overexpression induces the immune escape of tumor cells and is associated with a poor prognosis and enhanced tumor progression,” the authors wrote. “We realized that PVR may be a very important protein that human cancers use to hobble the immune system’s attack,” added Zang.
Moreover, PVRs appeared to inhibit T cell and NK cell activity by binding to a checkpoint protein called TIGIT—prompting efforts to interrupt the TIGIT-PVR pathway through the use of anti-TIGIT monoclonal antibodies. More than 100 clinical trials targeting TIGIT are now in progress worldwide. However, several clinical studies, including two large Phase III clinical trials, have recently failed to improve cancer outcomes.
The cancer-cell protein PVR has been found to have another binding partner on NK cells, known as KIR2DL5, which is a member of the human killer-cell, immunoglobin-like receptor (KIR) family. However, as the authors acknowledged, “The biology and therapeutic potential of the KIR2DL5-PVR pathway are largely unknown.” Zang further noted, “We hypothesized that PVR suppresses NK cell activity not by binding with TIGIT but by binding with the recently recognized KIR2DL5,” said Zang. To investigate this further, Zang and colleagues synthesized a monoclonal antibody targeting KIR2DL5 and carried out a series of in vitro and in vivo experiments.
In their newly reported paper, the investigators demonstrated that KIR2DL5 is a commonly occurring checkpoint receptor on the surface of human NK cells, which PVR cancer proteins use to suppress immune attack. “We found that KIR2DL5A mRNA was upregulated in several human solid tumors and hematopoietic malignancies by comparison with respective normal tissues,” they wrote. The results from further in vitro experiments demonstrated the presence of the immunosuppressive KIR2DL5-PVR pathway within the tumor microenvironment of various human cancers of bladder, kidney, breast, lung, liver, cerebrum, prostate, colon, esophagus, pancreas, uterus, and stomach, which, the team commented, may be exploited by tumors as “an immune-evasion mechanism.” The findings, they stated, “… support PVR as a primary ligand for KIR2DL5 to induce NK cell suppression and tumor immune evasion.”
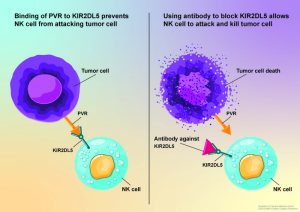
In studies involving humanized animal models of several types of human cancers, the researchers further confirmed that blocking the KIR2DL5/PVR pathway using their anti-KIR2DL5 monoclonal antibody allowed NK cells to attack and shrink human tumors and prolong animal survival.
“Markedly, the KIR2DL5 blockade reduced tumor growth and improved the overall survival across multiple NK cell-based humanized tumor models,” they wrote. “Taken together, these results demonstrated that blockade of KIR2DL5-PVR reinvigorated NK cell function and enhanced human NK cell-based antitumor immunity in vitro and in vivo … our findings unraveled the cellular and molecular mechanisms underlying the inhibitory function of the KIR2DL5-PVR pathway, supporting that blockade of the immunosuppressive KIR2DL5-PVR axis alone or combination with other therapies are new therapeutic strategies.”
Zang explained to GEN, “Currently there are more than 100 clinical trials targeting TIGIT worldwide, but two large Phase III clinical trials have recently failed this year. Our study revealed that KIR2DL5 bounds to PVR without competition with TIGIT, and KIR2DL5/PVR inhibited human NK cell function much better than TIGIT/PVR, so both receptors TIGIT and KIR2DL5 can function simultaneously and independently and that blockade of the TIGIT/PVR axis would still leave the KIR2DL5/PVR pathway intact. The combination of blockade of both KIR2DL5 and TIGIT may give better clinical therapeutic efficacy.”
“These preclinical findings raise our hopes that targeting the KIR2DL5/PVR pathway was a good idea and that the monoclonal antibody we’ve developed may be an effective immunotherapy,” he further commented in a press statement.
Zang has previously developed and patented more than 10 immune checkpoint inhibitors. One of those inhibitors, which Zang confirmed to GEN is a monoclonal antibody targeting the immune checkpoint Tim-3, is now being tested in China in Phase II clinical trials involving several hundred patients with advanced solid cancers (non-small cell lung cancer, small cell lung cancer, nasopharyngeal cancer, head and neck cancer, melanoma, lymphoma) or recurrent/refractory blood cancers (acute myeloid leukemia, myelodysplastic syndromes). Another immune checkpoint inhibitor developed by Zang will start to undergo evaluation next year in cancer clinical trials in the United States. He confirmed to GEN that this drug is again a monoclonal antibody against an as-yet undisclosed target, and will represent a first-in-class drug.
Einstein has filed a patent application for KIR2DL5/PVR immune checkpoint including antibody drugs, and looking to partner in further development and commercialization of the technology. Zang acknowledged, “Our drug would be first-in-class, so we need to find a partner to further develop and commercialize the technology … before we submit an investigational new drug (IND) application to the FDA.”