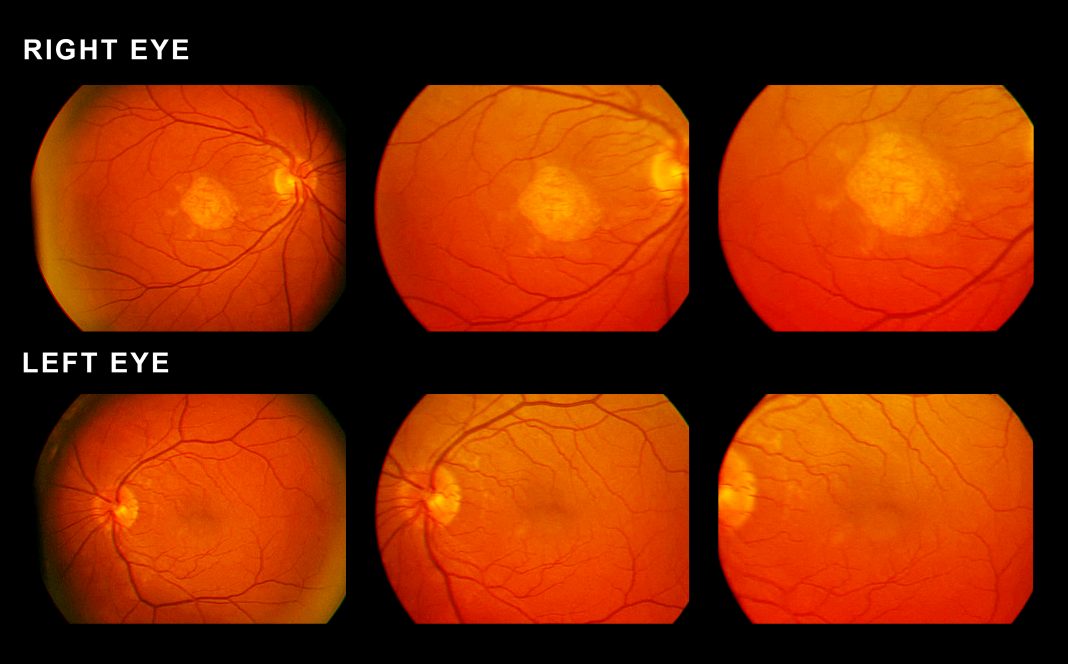
An international team of scientists has identified a protein which is strongly linked to the commonest cause of blindness in developed countries when its levels are raised in the blood. The discovery is a major step forward in the understanding of age-related macular degeneration, which affects 1.5 million people in the U.K. alone, according to the researchers.
The study (“Increased circulating levels of Factor H-Related Protein 4 are strongly associated with age-related macular degeneration”), carried out by the team from Universities of Manchester, Cardiff, London, Nijmegen, and the Manchester University NHS Foundation Trust, is published in Nature Communications.
“Age-related macular degeneration (AMD) is a leading cause of blindness. Genetic variants at the chromosome 1q31.3 encompassing the complement factor H (CFH, FH) and CFH related genes (CFHR1-5) are major determinants of AMD susceptibility, but their molecular consequences remain unclear. Here we demonstrate that FHR-4 plays a prominent role in AMD pathogenesis. We show that systemic FHR-4 levels are elevated in AMD (P-value = 7.1 × 10−6), whereas no difference is seen for FH. Furthermore, FHR-4 accumulates in the choriocapillaris, Bruch’s membrane and drusen, and can compete with FH/FHL-1 for C3b binding, preventing FI-mediated C3b cleavage,” write the investigators.
“Critically, the protective allele of the strongest AMD-associated CFH locus variant rs10922109 has the highest association with reduced FHR-4 levels (P-value = 2.2 × 10−56), independently of the AMD-protective CFHR1–3 deletion, and even in those individuals that carry the high-risk allele of rs1061170 (Y402H). Our findings identify FHR-4 as a key molecular player contributing to complement dysregulation in AMD.”
The FHR4 protein was found to be present at higher levels in the blood of patients with AMD compared to individuals of a similar age without the disease. The findings were confirmed in 484 patient and 522 control samples from two independent collections across Europe. Analyses of eyes donated for research after life also revealed the FHR4 protein was present in the AMD-affected parts of the eye
FHR4 was shown by the team to activate the complement system; over activation is a major causal factor of AMD. FHR4 is one of a group of proteins that regulate the complement system and the genes encoding these proteins are tightly clustered on chromosome 1, the largest human chromosome.
When the team investigated a set of genetic variants across the human genome, they found that genetic variants in this region on chromosome 1 determined the levels of FHR4 in the blood. And they found that the same genetic variants were associated with AMD. Paul Bishop, PhD, FRCOphth, and Simon Clark, DPhil, from the University of Manchester were part of the leadership team on the study. Bishop, who is also a consultant ophthalmologist at Manchester Royal Eye Hospital, said: “The combined protein and genetic findings provide compelling evidence that FHR4 is a critical controller of that part of the immune system which affects the eyes. We have shown that genetically determined higher blood FHR4 levels leads to more FHR4 in the eye which in turn increases the risk of the uncontrolled immune system response that drives the disease. So apart from improving understanding of how AMD is caused, this work provides a way of predicting risk of the disease by simply measuring blood levels of FHR4.”
The research also provides a new route to treatment by reducing the blood levels of FHR4 to restore immune system function in the eyes, according to Bishop, who added that “because treatments options for AMD are limited, this comprehensive understanding of the biology of AMD is a huge boost for scientists finding answers to a problem which causes untold misery for thousands of people in the U.K. alone.”
Clark, a specialist in the regulation of the complement system in health and disease, noted that “This study really is a step-change in our understanding of how complement activation drives this major blinding disease. Up until now, the role played by FHR proteins in disease has only ever been inferred. But now we show a direct link and, more excitingly, become a tangible step closer to identifying a group of potential therapeutic targets to treat this debilitating disease.”