The discovery that a tiny nematode worm exhibits fear-like responses akin to human anxiety could help scientists develop effective new drugs for treating conditions such as posttraumatic stress disorder and panic disorder, according to researchers at the Salk Institute for Biological Studies.
The Salk Institute team, headed by Sreekanth Chalasani, Ph.D., an associate professor at the Molecular Neurobiology Laboratory, found that the nematode worm Caenorhabditis elegans responds to chemicals released by its predator, another worm called Pristiochus pacificus, with fear-like responses, including turning around and actively moving away. Treating the C. elegans worms with the human antianxiety drug sertraline stopped these behaviors, suggesting that at least some of the neural and chemical pathways that the drug impacts on are conserved in evolution.
“For years, we thought that only advanced brains like those of mammals would have this complex reaction,” Dr. Chalasani says. “But our study is showing that a simple animal expresses something very much like fear.” The Salk Institute researchers, and colleagues at Cornell University, Worcester Polytechnic Institute, and the University of California, San Diego, report on their findings in Nature Communications, in a paper entitled “Predator-Secreted Sulfolipids Induce Defensive Responses in C. elegans.”
Animal survival is inextricably linked with the ability to detect dangers such as predators, and to respond appropriately. We are all familiar with the “fight or flight” response that grips us when frightened or anxious. These responses are often “hard-wired” into the genome of the prey. The authors write, “ …for example, mice reliably exhibit fear-like responses to cat odors despite not having encountered cats for hundreds of generations.” However, we all perceive and react differently to potentially dangerous situations, and drugs that have been developed to treat anxiety don’t work equally in all patients.
Previous research in both vertebrates and invertebrates indicates that sight, sound, and, most often, the olfactory sense, or smell, are involved in signaling between predators and prey. To investigate the neural circuits that might be involved in olfactory signaling, the researchers turned to the nematode worm, C. elegans, which has a fully mapped neural network comprising just 302 neurons.
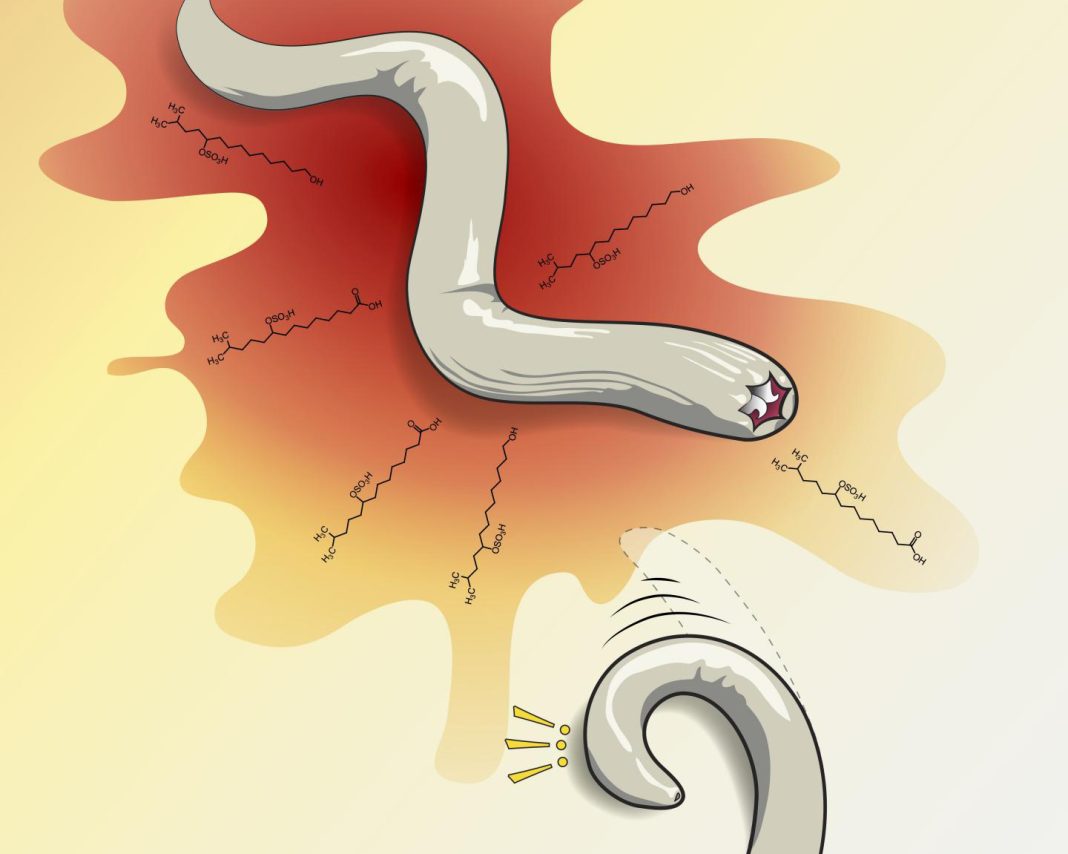
“For the past 30 or 40 years, scientists have used simpler animals to figure out how fear might work in humans,” says Chalasani. “The idea has been that if you could figure out which underlying signals in the brain are related to fear and anxiety, you could develop better drugs to block them.” The team’s studies found that C. elegans effectively turns tail and runs when exposed to a novel class of sulfolipid chemicals released by its natural predator, P. pacificus. The sulfolipids also caused the C. elegans worms to stop laying eggs, suggesting that predator cue-induced stress affects egg-laying behavior, the team notes. In fact, the worms failed to lay eggs even up to an hour after the P. pacificus chemicals had been withdrawn, an indicator of a longer-term anxiety-like response.
Interestingly, the P. pacificus suflolipids are structurally similar to sodium dodecyl sulfate (SDS), which the authors describe as “a potent C. elegans avoidance cue.” The team then tested all 12 pairs of amphid sensory neurons in the C. elegans to identify which neural circuit detected predator cue. (The amphid neurons put dendrites out into the nose of an animal to sense environmental changes.) The analyses showed that in the case of C. elegans the fear-triggering sulfolipids activated four redundant brain circuits. Animals lacking pairs of ASJ, ASH, ASI, or ADL neurons didn’t respond to predator cues, “indicating that C. elegans uses multiple sensory neurons to detect predators,” the authors write. “Detection of predator cue relies on a sensory neural circuit consisting of at least four different amphid neurons (ASI, ASH, ASJ and ADL). These neurons have well-described roles in detecting chemicals from the environment.…In contrast, animals missing any of the other 8 neuronal pairs showed normal responses suggesting that these neurons were not required for avoidance to predator cue.”
Interestingly, when the C. elegans worms were placed a solution containing the antianxiety drug sertraline, they didn’t display fear or anxiety-related behaviors on exposure to the sulfolipids. Further tests showed that the drug acted on gamma-aminobutyric acid (GABA) signaling in the RIS interneuron, which studies suggest is involved in controlling a sleep-like state in the tiny nematode. It's not yet known whether this is also the case in humans, but the finding does point to a potential pathway that might help understand why sertraline works in some people and not others.
“Sertraline has been shown to be particularly effective in alleviating human anxiety disorders and, classified as an SSRI [selective serotonin reuptake inhibitor], is thought to act in part by elevating serotonin levels at synapses,” the team states. “Our studies show that sertraline requires GABA, but not serotonin signaling, to exert its effects on C. elegans avoidance behavior.…Based on these results, we hypothesize that C. elegans evolved mechanisms to detect Pristionchus-released sulfolipids as a kairomone, and that the identified neuronal signaling circuitry is representative of conserved or convergent strategies for processing predator threats.”
“We hope the findings from this paper will contribute to the field by providing a broader picture of some of these signaling activities,” Chalasani concludes. “Our findings suggest that fear and anxiety are ancient and evolved much earlier than we originally thought. The pathways, nerves, circuits, and genes that we'll now be able to study in the worm should inform us about this process in humans.”