Scientists in the department of biomedical engineering at Duke University have revealed that exercising human muscles has an intrinsic ability to heal painful inflammation. The study focused on exploring how human skeletal muscle strength and structure are affected by a chemical messenger molecule, interferon gamma, typically elevated in inflammatory diseases.
The study is published in the journal Science Advances in an article titled, “Exercise Mimetics and JAK Inhibition Attenuate IFN-γ-induced Wasting in Engineered Human Skeletal Muscle.”
Earlier studies in humans and animals have shown exercise can help mitigate the effects of inflammation but the role of muscle fibers themselves in the process had not been explored nor their interactions with inflammation-instigating molecules, such as interferon gamma.
“Lots of processes are taking place throughout the human body during exercise, and it is difficult to tease apart which systems and cells are doing what inside an active person,” said Nenad Bursac, PhD, professor of biomedical engineering at Duke. “Our engineered muscle platform is modular, meaning we can mix and match various types of cells and tissue components if we want to. But in this case, we discovered that the muscle cells were capable of taking anti-inflammatory actions all on their own.”
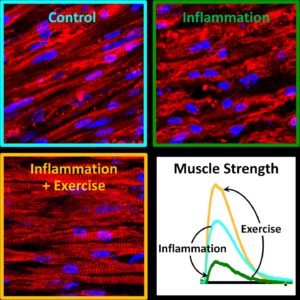
Lab-grown muscle bundles fluoresce when their calcium levels spike—a proxy for measuring their strength. The muscle bundles that were electrically stimulated to approximate exercise during inflammation (right) flash just as robustly as normal muscle bundles (left) and much more brightly than unexercised muscles after chronic inflammation (middle). [Zhaowei Chen, Duke University]
These findings were facilitated through the development of a first-of-its-kind research platform that Bursac’s laboratory has been developing for nearly a decade, where human muscle bundles were engineered in the lab and electrically stimulated to mimic exercise.
Short-term or acute inflammation, a physiological response to infection or injury usually accompanied by redness, heat, and swelling, is an innate immune response that clears away cellular debris and helps muscles rebuild. However, at times the immune system goes off-kilter and the inflammatory response magnifies or extends over prolonged periods, leaving surrounding tissues weak and damaged. For instance, in diseases such as rheumatoid arthritis and sarcopenia, prolonged uncontrolled inflammation causes muscle to waste away.
“We know that chronic inflammatory diseases induce muscle atrophy, but we wanted to see if the same thing would happen to our engineered human muscles grown in a Petri dish,” said Zhaowei Chen, PhD, a postdoctoral researcher in Bursac’s laboratory and first author of the paper. “Not only did we confirm that interferon gamma primarily works through a specific signaling pathway, we showed that exercising muscle cells can directly counter this pro-inflammatory signaling independent of the presence of other cell types or tissues.”
The researchers then applied interferon gamma again, but this time also electrically stimulated the muscle with a pair of electrodes that caused it to contract, mimicking an exercise regime. While they expected the procedure to induce some muscle growth, as shown in their previous studies, they were surprised to observe that the muscle did not get smaller or weaker at all.
“This is the first study to explore direct and specific effects of interferon gamma on human skeletal muscle function and to demonstrate the existence of a novel myofiber-autonomous, anti-inflammatory mechanism of muscle exercise involving the JAK/STAT1 pathway,” noted the authors.
Besides its well-established growth-promoting and strengthening effects, the exercise-mimicking electrical stimulation down-regulated the JAK (Janus kinase)/STAT1 (signal transducer and activator of transcription 1) signaling pathway that is typically upregulated by interferon gamma. The authors showed that two drugs used to treat rheumatoid arthritis, tofacitinib and baricitinib, which block the same pathway, had the same anti-inflammatory effect.
“When exercising, the muscle cells themselves were directly opposing the pro-inflammatory signal induced by interferon gamma, which we did not expect to happen,” said Bursac. “These results show just how valuable lab-grown human muscles might be in discovering new mechanisms of disease and potential treatments. There are notions out there that optimal levels and regimes of exercise could fight chronic inflammation while not overstressing the cells. Maybe with our engineered muscle, we can help find out if such notions are true.”
Bursac and his team anticipate the future use of their myobundle platform to study human inflammatory disease and anti-inflammatory therapies.




