Scientists from TARA Biosystems and GlaxoSmithKline (GSK) published data demonstrating that TARA’s engineered heart-on-a-chip system replicated drug responses found in adult humans. The study (“Engineered Cardiac Tissues Generated in the Biowire™ II: A Platform for Human-Based Drug Discovery”), which appears in the Journal of Toxicological Sciences, shows TARA’s 3D-cardiac tissue platform can predict how human hearts will respond to a wide range of drugs, something that has been a challenge in preclinical models until now, according to the research team.
“Recent advances in techniques to differentiate human induced pluripotent stem cells (hiPSCs) hold the promise of an unlimited supply of human-derived cardiac cells from both healthy and disease populations. That promise has been tempered by the observation that hiPSC-derived cardiomyocytes (hiPSC-CMs) typically retain a fetal-like phenotype, raising concern about the translatability of the in vitro data obtained to drug safety, discovery, and development studies,” the investigators wrote.
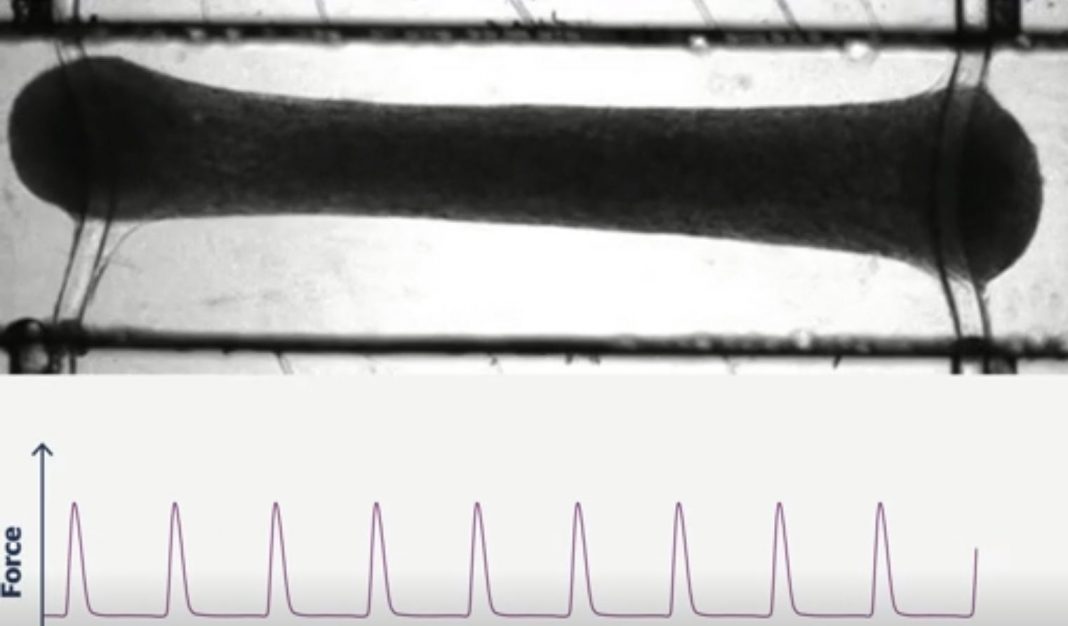
“The Biowire II platform was used to generate 3D engineered cardiac tissues (ECTs) from hiPSC-CMs and cardiac fibroblasts. Long-term electrical stimulation was employed to obtain ECTs that possess a phenotype like that of adult human myocardium including a lack of spontaneous beating, the presence of a positive force-frequency response from 1-4Hz, and prominent post-rest potentiation.
“Pharmacology studies were performed in the ECTs to confirm the presence and functionality of pathways that modulate cardiac contractility in humans. Canonical responses were observed for compounds that act via the β-adrenergic/cAMP-mediated pathway, e.g., isoproterenol and milrinone; the L-type calcium channel, e.g., FPL64176 and nifedipine; and indirectly effect intracellular Ca2+ concentrations, e.g., digoxin. Expected positive inotropic responses were observed for compounds that modulate proteins of the cardiac sarcomere, e.g., omecamtiv mecarbil and levosimendan.
“ECTs generated in the Biowire II platform display adult-like properties and have canonical responses to cardiotherapeutic and cardiotoxic agents that affect contractility in humans via a variety of mechanisms. These data demonstrate that this human-based model can be used to assess the effects of novel compounds on contractility early in the drug discovery and development process.”
“The cardiac tissues generated in the study are the first to provide high-fidelity human drug responses and demonstrate the potential for evaluating the safety and efficacy of new therapies in preclinical development,” said Michael P. Graziano, PhD, study author and chief scientific officer of TARA Biosystems.
Source: TARA Biosystems
Cardiac toxicity is responsible for almost half of new medicine recalls. Regulations mandate that new drugs undergo a rigorous cardiac safety assessment before human testing but predicting how human hearts will respond to potential drug toxicity has been difficult. Traditional in vitro systems and animal models do not fully capture the physiology of the human heart.
hiPSCs hold great promise as a foundation to bridge the human translation gap. However, experimental models which rely on iPSCs alone lack relevant physiological hallmarks and drug responses seen in human heart muscle.
TARA leverages the power of iPSCs and subjects them to a rigorous maturation process on its patented Biowire II system, producing 3D human cardiac tissues called Cardiotype™ tissues, explained Graziano. In a study published earlier this year in Cell, TARA scientific founders validated the ability of the Biowire II platform to create physiologically relevant human cardiac tissues, he noted, adding that unlike models that rely solely on iPSCs, researchers can measure the force with which Cardiotype tissues contract, which better reflects human heart physiology by offering a measure of how well the heart can pump blood. The research also showed how the platform could be used to model different heart diseases by using iPSCs from patients.
In the current study, scientists from GSK worked with TARA to select a panel of clinically relevant drugs with known efficacy and toxicity profiles in the heart, for screening in the Cardiotype platform. These drugs, and their concentrations, were chosen to assess the predictivity of the Cardiotype assay in comparison to current methods for preclinical drug screening in pharmaceutical R&D.
The tissues responded to the range of cardiotherapeutic and cardiotoxic drugs selected as expected, replicating for the first time the human-like response to the drugs that other laboratory models failed to capture, said Graziano. The researchers also confirmed the findings at the molecular level, showing that the drugs were acting along the same molecular pathways seen in human heart tissue.
“At TARA we are proud to tackle cardiac safety in a meaningful way that will ultimately improve the quality of new medicines delivered to patients,” said Misti Ushio, PhD, CEO of TARA Biosystems. “By using TARA’s cardiac engineered tissue, drug development companies are able to obtain human relevant data earlier in the drug development cycle.”