Radiation is one of the most common treatments for cancer. Radiation therapy uses high-energy particles or waves, such as x-rays, gamma rays, electron beams, or protons, to destroy or damage cancer cells. It makes small breaks in the DNA inside cells to keep cancer cells from growing and dividing and cause them to die. Nearby normal cells can also be affected by radiation, but most recover and go back to working the way they should. Unfortunately, there are varieties of cancer that become resistant to these therapies, and in some cases, these radioresistant cancers can become more invasive following treatment, worsening the prognosis for the patient.
Now, scientists from the Global Center for Biomedical Science and Engineering, a collaboration between Hokkaido University in Japan, and Stanford University, have discovered the mechanism by which molecules called Arl8b and BORC cause increased invasiveness and metastasis in radioresistant cancer cells following radiotherapy.
Their findings were published in the journal Communications Biology in a paper titled, “Lysosomal trafficking mediated by Arl8b and BORC promotes invasion of cancer cells that survive radiation.”

[Global Center for Biomedical Science and Engineering.]
Previous work and studies have shown that vesicle trafficking, including endocytosis, recycling, and the exocytosis of proteins and organelles, plays an essential role in cancer invasion. Recent studies have highlighted the important roles lysosomes play in tumor biology. The researchers confirmed that the trafficking of lysosomes was upregulated in the cancer cells following radiotherapy, enhancing the secretion of enzymes that degrade the connective material surrounding them, and therefore increasing the invasiveness of the cancer cells. They further investigated the molecular mechanisms behind this activity and determined that a regulatory molecule, Arl8b, is primarily responsible for this process.
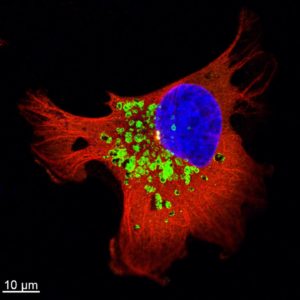
The findings suggest that lysosomes can be targeted for therapy of highly metastatic cancer, and may lead to the development of new strategies to combat radioresistant cancer cells and improve cancer treatment.