An international research team has used CRISPR-Cas9 gene editing to render induced pluripotent stem cells (iPSCs) invisible to the immune system, a bioengineering sleight of hand that initial in vivo tests suggest could open the door to generating stem cells for universal transplantation. The new approach, developed by scientists at the University of California, San Francisco (UCSF), involves disabling two histocompatibility complex genes, and overexpressing the transmembrane protein CD47 on iPSCs. In vivo tests suggested that engineered human iPSC-derived cardiac cells were undetected by the immune system in mouse recipients, and showed evidence of rudimentary tissue differentiation.
“This is the first time anyone has engineered cells that can be universally transplanted and can survive in immunocompetent recipients without eliciting an immune response,” said Tobias Deuse, MD, the Julien I.E. Hoffman, MD, endowed chair in cardiac surgery at UCSF, who led the research, and is lead author of the team’s published paper in Nature Biotechnology. “Our technique solves the problem of rejection of stem cells and stem cell-derived tissues, and represents a major advance for the stem cell therapy field. The researchers’ paper is titled, “Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients.”
iPSCs are a type of stem cell generated by reprogramming mature cells such as skin or fat cells into an undifferentiated state. These undifferentiated cells can then be grown in the laboratory and triggered to differentiate into desired cell types. The technology could feasibly be used to generate iPSCs from patients’ own cells, which could then be transplanted back into the same patient for cell therapy. Importantly, because these iPSCs are derived from the patient—autologous iPSCs—there would be no rejection issues, which is a major drawback associated when transplanted cells, tissues, or organs are not the patient’s own.
“Scientists often tout the therapeutic potential of pluripotent stem cells, which can mature into any adult tissue, but the immune system has been a major impediment to safe and effective stem cell therapies,” Deuse noted. The only option is to administer antirejection drugs, but they have major drawbacks. “We can administer drugs that suppress immune activity and make rejection less likely,” added professor of surgery Sonja Schrepfer, MD, PhD, the study’s senior author, who at the time of the research was director of the UCSF Transplant and Stem Cell Immunobiology (TSI) Lab. “Unfortunately, these immunosuppressants leave patients more susceptible to infection and cancer.”

“There are many issues with iPSC technology, but the biggest hurdles are quality control and reproducibility,” Deuse added. “We don’t know what makes some cells amenable to reprogramming, but most scientists agree it can’t yet be reliably done. Most approaches to individualized iPSC therapies have been abandoned because of this.”
Deuse and Schrepfer reasoned that it might be possible to sidestep the problems associated with generating patient-specific iPSCs, by creating universal iPSCs that could be used for any patient without causing any rejection issues. Taking hints from the immunologically more tolerant pregnant maternal immune system, the researchers used CRISPR technology to inactivate two major histocompatibility (MHC) class I and II genes, and upregulate a third gene, CD47. Cell surface MHC proteins play key roles in presenting foreign molecules from invading pathogens. Although cells lacking these genes may be more tolerant of foreign proteins, they then become the targets of the immune system’s natural killer (NK) cells.
Schrepfer’s team then worked with Lewis Lanier, PhD, study co-author, chair of UCSF’s department of microbiology and immunology, and an expert in signals that activate and inhibit NK cell activity, to determine how it might be possible to prevent NK cell attack on the MHC gene-deprived engineered iPSCs. They found that the cell surface protein CD47, which effectively acts as a “do not eat me” signal to macrophages, also inhibits NK cell activity. The researchers then used a lentiviral vector to deliver extra copies of the CD47 gene to the MHC gene-deficient iPSCs.

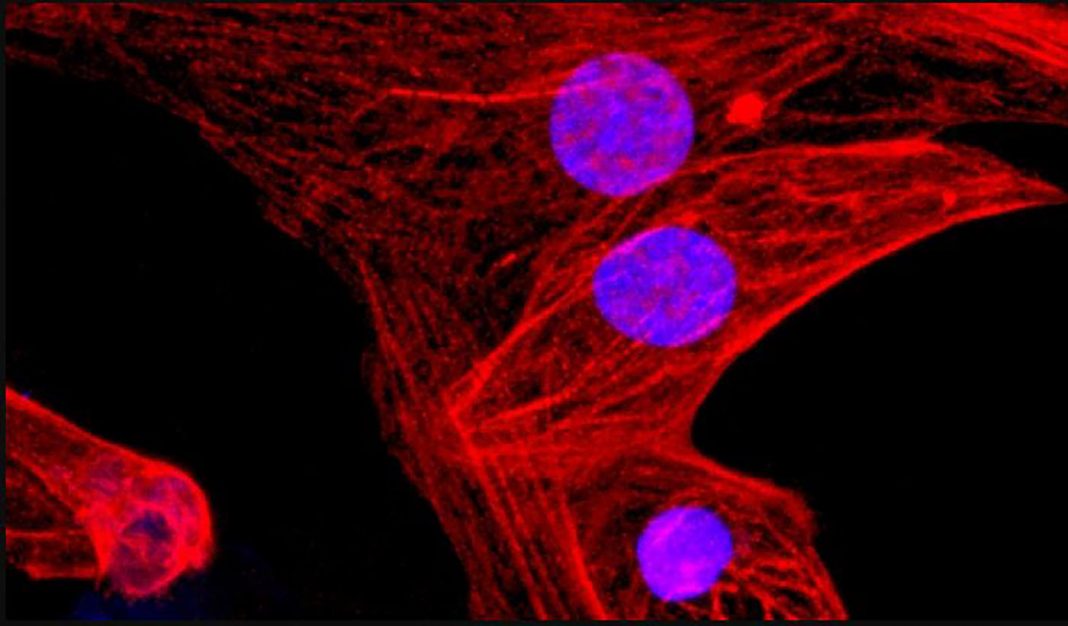
Hypoimmunogenic iPSCs also retained their pluripotent stem cell capacity, as well as their ability to differentiate. The team successfully derived various types of heart cells from the triple engineered human iPSCs, and transplanted the cells into humanized mice. The iPSC-derived cardiac cells survived over the long term, and even began forming fundamental blood vessels and heart muscle, pointing to the potential use of such stem cells for cardiac cell therapy.
“Endothelial cells, smooth muscle cells, and cardiomyocytes derived from hypoimmunogenic mouse or human iPSCs reliably evade immune rejection in fully MHC-mismatched allogeneic recipients and survive long-term without the use of immunosuppression,” the team wrote. “Thus, the generation of universal hypoimmunogenic iPSCs that can be differentiated into the main components of cardiac tissue and achieve long-term survival in a fully allogeneic recipient without any immunosuppression may help to develop universal cell products to treat heart failure … These findings suggest that hypoimmunogenic cell grafts can be engineered for universal transplantation.”
“Our technique can benefit a wider range of people with production costs that are far lower than any individualized approach,” Deuse stated. “We only need to manufacture our cells one time and we’re left with a product that can be applied universally.”