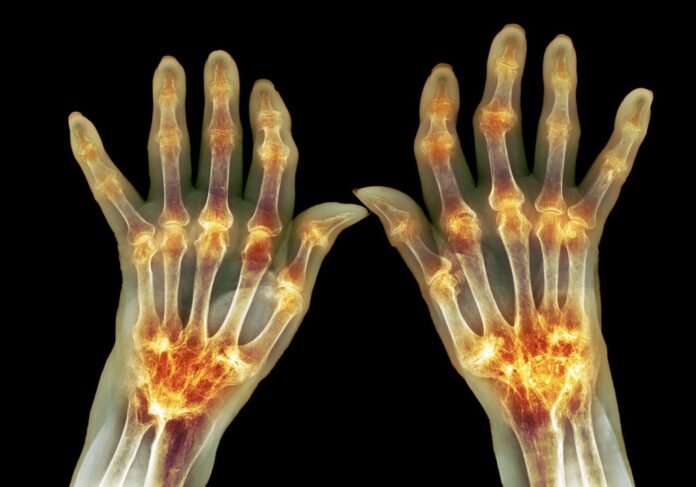
Researchers at Washington University School of Medicine in St. Louis have used CRISPR-Cas9 genome editing to engineer induced pluripotent stem cells (iPSCs) that can be implanted subcutaneously to deliver an anticytokine biologic drug in response to inflammation caused by rheumatoid arthritis. When implanted into a mouse model of rheumatoid arthritis the engineered cells automatically sensed and responded to inflammatory cytokines, and produce therapeutic levels of the drug, which reduced inflammation and also prevented bone erosion.
“Doctors often treat patients who have rheumatoid arthritis with injections or infusions of anti-inflammatory biologic drugs, but those drugs can cause significant side effects when delivered long enough and at high enough doses to have beneficial effects,” said senior investigator Farshid Guilak, PhD, the Mildred B. Simon Professor of Orthopedic Surgery. “We used CRISPR technology to reprogram the genes in stem cells. Then we created a small cartilage implant by seeding the cells on woven scaffolds, and we placed them under the skin of mice. The approach allows those cells to remain in the body for a long time and secrete a drug whenever there is a flare of inflammation.”
Guilak and colleagues report on their studies in Science Advances, in a paper titled, “A genome-engineered bioartificial implant for autoregulated anticytokine drug delivery.”
Disease-modifying anti-rheumatic biologic drugs have revolutionized the treatment of autoimmune diseases, the authors wrote, but about 40% of patients with rheumatoid arthritis (RA)—a disease that affects about 1.3 million adults in the U.S.—fail to respond to treatment. “Moreover, anti-rheumatic biologic drugs suppress the immune system, predisposing patients to substantial adverse effects (AEs), such as increased risk of infection.”
Biologic drugs are generally designed to target several inflammatory cytokines and pathways, including interleukin-1 (IL-1), IL-6, and tumor necrosis factor-α (TnF-α). But while the severity of RA disease may fluctuate over time, the drugs tend to be administered continuously, and at high concentrations, regardless of cytokine levels or RA symptoms. “The development of specific therapeutic strategies that can sense and respond to endogenous levels of inflammatory mediators could overcome some of the challenges observed with constant delivery of cytokine inhibitors and mitigate unwanted AEs,” the team continued.
“The idea of delivering such drugs essentially on demand in response to arthritis flares is extremely attractive to those of us who work with arthritis patients, because the approach could limit the adverse effects that accompany continuous high-dose administration of these drugs,” commented co-author Christine Pham, MD, director of the Division of Rheumatology and the Guy and Ella Mae Magness Professor of Medicine.
Guilak, a co-director of the Washington University Center of Regenerative Medicine, and his team had previously developed a scaffold that can be coated with stem cells and then implanted into joints to form cartilage. This strategy allows the researchers to implant the engineered cartilage cells in such a way that they don’t drift away after a few days, and they can survive for months or longer.
The Guilak lab also previously built so-called SMART cartilage cells (Stem cells Modified for Autonomous Regenerative Therapy) using CRISPR-Cas9 technology to alter genes in those cells, so that when the genes in the cartilage are activated by inflammation, they secrete drugs in response.
For the newly reported study, Guilak’s team combined these two strategies to generate a new treatment approach for rheumatoid arthritis. To develop their implantable cell-based therapy, the researchers used CRISPR-Cas9 genome editing technology to engineer induced pluripotent stem cells that secrete an anticytokine biologic drug in response to inflammation. The drug reduces inflammation in joints by binding to the cytokine IL-1, which can promote inflammation in arthritis by activating inflammatory cells in a joint. “We used CRISPR-Cas9 genome editing of iPSCs to create a synthetic gene circuit that senses changing levels of endogenous inflammatory cytokines to trigger a proportional therapeutic response,” the team explained.
“The cells sit under the skin or in a joint for months, and when they sense an inflammatory environment, they are programmed to release a biologic drug,” said Guilak, who is also director of research at Shriners Hospitals for Children — St. Louis. The anticytokine biologic drug was similar to the immunosuppressant drug anakinra, which binds to IL-1 and blocks its activity. Interestingly, the drug isn’t used frequently to treat rheumatoid arthritis because it has a short half-life.
“Although IL-1 receptor antagonist (IL-1Ra) has been shown to mitigate disease in animal models of RA and slows joint damage (assessed radiographically) in humans, it is not routinely used as a disease-modifying biologic in RA because of its short half-life and modest effects,” the team noted.
But in their study in mice with the implanted cells, the drug reduced inflammation and prevented the type of bone damage that is often seen in rheumatoid arthritis. “Our results show that dynamic, autoregulated delivery of IL-1Ra mitigated inflammation, pain, and structural damage in K/BxN arthritis that was superior to conventional disease-modifying antirheumatic drugs (DMARDs) in this context,” the authors noted. “This therapeutic approach completely abrogated bone erosions as compared to conventional disease-modifying drugs and biologics, which is yet to be achieved by daily subcutaneous injection of IL-1Ra (anakinra).”
“We focused on bone erosion because that is a big problem for patients with rheumatoid arthritis, which is not effectively treated by current biologics” said co-first author Yunrak Choi, MD, a visiting orthopedic surgeon in the Guilak lab. “We used imaging techniques to closely examine bones in the animals, and we found that this approach prevented bone erosion. We’re very excited about this advance, which seems to meet an important unmet clinical need.”
Using CRISPR-Cas9 gene editing it is feasible that cells can be programmed to make different types of drug, which means that if one arthritis drug works better than another in a particular patient, it may be possible to engineer cartilage cells to make personalized treatments. The strategy also has great potential to treat other inflammatory arthritis conditions, including juvenile arthritis, a condition that affects more than 300,000 children in the U.S.
“Many arthritis patients have to self-administer these drugs, giving themselves injections daily, weekly or biweekly, while others go to a doctor’s office every few months to receive an infusion of one of these biologics, but in this study, we’ve demonstrated that we can make living tissue into a drug-delivery system,” said Kelsey H. Collins, PhD, a postdoctoral research associate in Guilak’s lab and co-first author of the study. “These cells can sense problems and respond by producing a drug. The authors concluded, “The present cell-based anticytokine therapy dynamically senses and responds to the environment, so this feature can help overcome the short half-life and rapid clearance, as IL-1Ra is being synthesized continuously in response to stimulus, rather than being delivered in a single bolus that is rapidly cleared.”
“This approach also helps us understand why certain biologics may have limited effects in inflammatory arthritis,” Collins further noted. “It’s not because they don’t bind to the right target but likely because an injected drug is short-lived compared to the automatically controlled levels of drug released by implanted SMART cells.”
The researchers are continuing to experiment with CRISPR-Cas9 and stem cells, even engineering cells that might manufacture more than one drug to respond to different triggers for inflammation. “By leveraging the flexibility of iPSCs as a cell source, this approach can be readily expanded to generate therapeutic cells that could be broadly applied and differentiated into any cell type in the future, allowing for additional applications to address a variety of chronic diseases and tissue types where defined biologic targets have been identified,” they suggested. “Ongoing work is interrogating other biomaterials and synthetic gene circuits for further tuning of the cellular responses in other chronic inflammatory disease models.”