The National Institutes of Health (NIH) is hoping to push COVID-19 testing to the next level by funding research groups who are innovating new testing technologies. A new initiative was recently announced that aims at speeding innovation, development, and commercialization of COVID-19 testing technologies. With a $1.5 billion investment from federal stimulus funding, the newly launched Rapid Acceleration of Diagnostics (RADx) initiative will infuse funding into early innovative technologies to speed development of rapid and widely accessible COVID-19 testing.
As part of this initiative, NIH is urging all scientists and inventors with a rapid testing technology to compete in a national COVID-19 testing challenge for a share of up to $500 million over all phases of development. The technologies will be put through a highly competitive, rapid three-phase selection process to identify the best candidates for at-home or point-of-care tests for COVID-19. Finalists will be matched with technical, business, and manufacturing experts to increase the odds of success. If certain selected technologies are already relatively far along in development, they can be put on a separate track and be immediately advanced to the appropriate step in the commercialization process. The goal is to make millions of accurate and easy-to-use tests per week available to all Americans by the end of summer 2020, and even more in time for the flu season.
“We need all innovators, from the basement to the boardroom, to come together to advance diagnostic technologies, no matter where they are in development,” said Francis S. Collins, MD, PhD, director, NIH. “Now is the time for that unmatched American ingenuity to bring the best and most innovative technologies forward to make testing for COVID-19 widely available.”
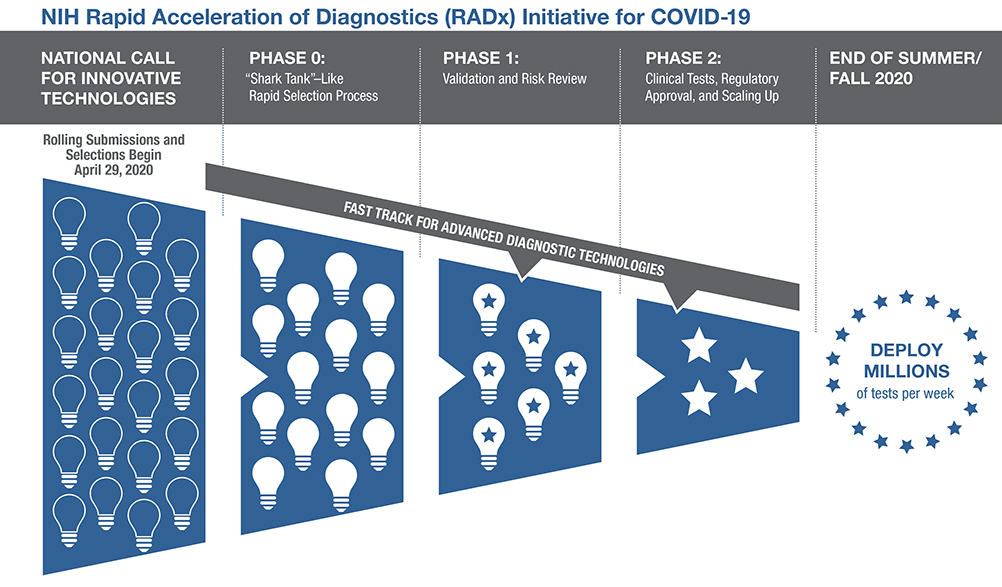
NIH will also seek opportunities to move more advanced diagnostic technologies swiftly through the development pipeline toward commercialization and broad availability. NIH will work closely with the FDA, CDC, and the Biomedical Advanced Research and Development Authority (BARDA) to advance these goals.
The stimulus investment supercharges NIH’s research efforts already underway focused on prevention and treatment of COVID-19, including the recently announced planned Accelerating COVID-19 Therapeutic Interventions and Vaccines public-private partnership to coordinate the international research response to the pandemic.
“Americans are innovators and makers,” said Bruce J. Tromberg, PhD, director of NIH’s National Institute of Biomedical Imaging and Bioengineering (NIBIB). “We need American tech experts, innovators, and entrepreneurs to step up to one of the toughest challenges we’ve faced as a country, to help get us safely back to public spaces.”
While diagnostic testing has long been a mainstay of public health, newer technologies offer patient- and user-friendly designs, mobile-device integration, reduced cost, and increased accessibility both at home and at the point of care. RADx will expand the Point-of-Care Technologies Research Network (POCTRN) established several years ago by NIBIB. The network will use a flexible, rapid process to infuse funding and enhance technology designs at key stages of development, with expertise from technology innovators, entrepreneurs, and business leaders across the country. POCTRN supports hundreds of investigators from multiple universities and businesses through five technology hubs:
- Emory University/Georgia Institute of Technology, Atlanta, GA
- Johns Hopkins University, Baltimore, MD
- Northwestern University, Evanston, IL
- University of Massachusetts Medical School, Worcester, MA
- Consortia for Improving Medicine with Innovation & Technology (CIMIT) at Harvard Medical School/Massachusetts General Hospital, Boston, MA
Led by the Coordinating Center at CIMIT, the network has assembled expert review boards covering scientific, clinical, regulatory, and business domains that will rapidly evaluate technology proposals. In order to roll out new products starting at the end of summer/fall 2020, a rapid, parallel process will allow quick throughput of projects. Projects will be assessed at each milestone and must demonstrate significant progress to receive continued support.



