Activating an immune signaling pathway known as STING, which is best known for fighting viral and bacterial infections, can boost the ability of genetically engineered T cells to eradicate breast cancer in mice, according to a newly reported study by researchers at the University of North Carolina (UNC). Their findings, reported in the Journal of Experimental Medicine, suggest that chimeric antigen receptor (CAR) T cells, which are already used to treat certain blood cancers in humans, may also be successful against solid tumors if combined with other immunotherapeutic approaches.
“We know that CAR T cells are safe for patients with solid tumors but so far they have not been able to cause significant tumor regression in the overwhelming majority of people treated,” said Jonathan S. Serody, MD, the Elizabeth Thomas professor of medicine, microbiology, and immunology and director of the immunotherapy program at UNC Lineberger Comprehensive Cancer Center. “Now we may have a new approach to make CAR T cells work in solid tumors, which we think could be a game-changer for therapies aimed at an appreciable number of cancers.” In their published paper, which is titled, “STING agonist promotes CAR T cell trafficking and persistence in breast cancer,” corresponding author Serody, and colleagues, concluded: “To our knowledge, these data are the first to demonstrate the function of a STING agonist in altering the trafficking properties of adoptive T cellular products.”

CAR T cells are a type of white blood cell that has been genetically engineered to recognize and attack cancer cells expressing specific proteins on their surface. CAR T cell treatments have been successfully used to treat patients with B cell lymphomas, and are currently undergoing clinical trials for the treatment of many other types of blood cancer. “However, the clinical activity of CAR T cells in patients or animal models with solid tumors has been modest,” said Serody, who is director of the cellular therapy program at the University of North Carolina School of Medicine.
CAR T cells may be less effective against solid tumors because they have to migrate into tumors and then survive long enough to kill all of the tumor cells. Moreover, the cells and molecules surrounding tumors are often immunosuppressive, activating an immune checkpoint that causes the CAR T cells to lose their activity.
When used to treat patients with non-solid tumors, such as lymphomas, CAR T cells home in on bone marrow and other organs that make up the lymphatic system. But for solid tumors, such as breast cancer, that is usually not the case. And even if they do migrate to the tumor, they don’t persist and expand well there, due to the nature of the microenvironment surrounding such tumors, noted Serody. “Potential barriers to CAR T cell efficacy against solid tumors include the suboptimal migration and persistence of CAR T cells in the tumor microenvironment (TME), impaired function mediated by the immunosuppressive TME, and CAR T cell exhaustion,” the authors explained.
For their newly reported research, Serody and colleagues looked for ways to direct lab-expanded cells to the sites of solid tumors. They focused on generating CAR T cells from Th17 and Tc17 cells, which are known to have longer persistence in the microenvironment that surrounds a tumor, in part due to their better survival capabilities. “Th17 cells and their counterpart Tc17 cells (CD8+ T cells that express IL-17A) have longer persistence in the TME, in part due to their better survival in vivo,” the team noted.
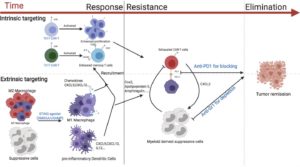
The researchers tested several strategies to boost the effectiveness of CAR T cells in a mouse model of breast cancer. Aiming to increase accumulation of the Th17 and Tc17 cells near solid tumors, they turned to two small molecules—the stimulator of interferon genes (STING) agonists—DMXAA and cGAMP, which can activate an immune response.
The STING pathway is an immune cell signaling pathway that normally induces inflammation in response to invading viruses or bacteria. In their experimental setting, the researchers showed that activating the STING pathway created a proinflammatory environment within the mouse tumors, improving the CAR T cells’ ability to accumulate and attack the tumor cells. “The most compelling finding was the ability of the STING agonists, DMXAA and c-GAMP, given at a site remote of the tumor, to promote CAR T cell recruitment and persistence at the tumor site,” the team stated. “CAR T cells generated from Th/Tc17 cells given with the STING agonists DMXAA or cGAMP greatly enhanced tumor control …”.
The accumulation was particularly great when the mice were infused with CAR T cells that produce the immune signaling molecule IL-17A, compared with CAR T cells generated using standard techniques.
Serody and colleagues determined that the CAR T cells’ attack could be sustained for longer periods if the mice were also treated with therapeutic antibodies that deplete immunosuppressive cells from the tumor environment and prevent the immune checkpoint from deactivating the CAR T cells. The combination of all these approaches led to the complete eradication of breast tumors. “…sustained tumor regression was accomplished only with the addition of anti–PD-1 and anti–GR-1 mAb to Th/Tc17 CAR T cell therapy given with STING agonists,” the investigators wrote.
The team suggested that their findings point to a “viable strategy” for boosting CAR T activity in solid tumors. And while DMXAA, which worked well in the investigator’s mouse studies, has not provided benefit in human clinical trials as it does not activate human STING, the second STING agonist, cGAMP, does activate human STING and is known to boost the human immune system.
“cGAMP is in clinical trials for the treatment of patients with cancer, there are multiple ongoing clinical trials using approaches to inhibit immunosuppressive cells for patients with malignant disease, and there are clinical trials currently evaluating the combination of CAR T cells with immune checkpoint blockade,” Serody said. “Together therefore, our data suggest a viable strategy for boosting CAR T activity in solid tumors … We hope to be able to study cGAMP in humans fairly soon. We will look to see if we can produce improvements in the treatment of head and neck cancers first, and if that proves promising, move into other forms of cancer by using CAR T cells generated by one of our colleagues here at UNC.”