A new study headed by researchers at Massachusetts General Hospital has linked heightened stress-related activity in the amygdala region of the brain with an increased risk of a rare and sometimes fatal heart condition known as ‘broken heart syndrome,’ or, more correctly, Takotsubo syndrome (TTS). Findings from the imaging study suggest that the greater the activity in nerve cells in the amygdala, the sooner TTS can develop. The researchers say their study indicates that developing strategies—such as drug treatments or other stress-reduction techniques—aimed at lowering stress-related activity in the amygdala could help to reduce the risk of developing TTS.
Tawakol and colleagues report on their study in European Heart Journal, in a paper titled, “Stress-associated neurobiological activity associates with the risk for and timing of subsequent Takotsubo syndrome.”
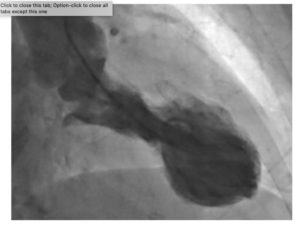
Takotsubo syndrome (TTS) is an acute, usually reversible heart failure syndrome that is often triggered by acute emotional or physical stressors,” the authors explained. The disorder is characterised by a sudden temporary weakening of the heart muscles that causes the left ventricle of the heart to balloon out at the bottom while the neck remains narrow, creating a shape resembling a Japanese octopus trap, from which it gets its name. Since this relatively rare condition was first described in 1990, evidence has suggested that it is typically triggered by episodes of severe emotional distress, such as grief, anger or fear, or reactions to happy or joyful events. Patients develop chest pains and breathlessness, and it can lead to heart attacks and death. TTS is more common in women with only 10% of cases occurring in men. However, as the authors pointed out, “although an underlying link between the brain and heart has long been proposed as a critical factor in the development of TTS, the underlying mechanisms require further clarification.”
In fact, they continued, functional magnetic resonance imaging (fMRI) studies have demonstrated altered neural connectivity in several stress-associated regions of the brain, including the amygdala, among individuals with prior TTS. “However, it remains unknown whether the observed alterations in neural activity predate TTS, or if they develop as a consequence of the syndrome.”
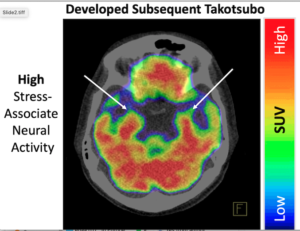
The amygdala is the part of the brain that controls emotions, motivation, learning and memory. It is also involved in the control of the autonomic nervous system and regulating heart function. In the first study to look at brain scans using F-fluorodeoxyglucose positron emission tomography/computed tomography (PET-CT) to assess brain activity before TTS develops, Tawakol and colleagues analyzed data on 104 people with an average age of 68 years, 72% of whom were women. “We tested the hypotheses that (i) heightened AmygA precedes development of TTS and (ii) those with the highest AmygA develop the syndrome earliest,” they wrote.
The patients had undergone scans at Massachusetts General Hospital (Boston, USA) between 2005 and 2019. Most of them had the scans to see if they had cancer and the scans also assessed the activity of blood cells in bone marrow. The researchers matched 41 people who went on to develop TTS between six months and five years after the scan, with 63 who did not.
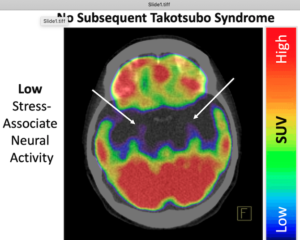
For example, individuals who had the highest amygdalar activity developed TTS within a year after imaging, while those with intermediate values developed TTS several years later. Among the 41 patients who developed TTS, the average interval between the scan and TTS was 0.9 months, whereas among the control group of 63 patients, the average interval between the scan and last follow-up or death was 2.9 years. The interval between the scan, the onset of TTs, last follow-up or death was an average (median) of 2.5 years for the 104 patients.
The authors concluded, “Together, the findings of the current study thus provide insights into one potential mechanism that may contribute to the ‘heart–brain connection’ in TTS by suggesting that chronically heightened stress-associated neurobiological activity (i.e. the ratio of amygdalar to regulatory activity) may potentially impact both the risk for and timing of subsequent TTS … This heightened neurobiological activity is present years before the onset of TTS and may impact the timing of the syndrome. Accordingly, heightened stress-associated neural activity may represent a therapeutic target to reduce stress-related diseases, including TTS.”
“It was notable that among the 41 patients who developed TTS, the top 15% with the very highest amygdalar activity developed TTS within a year of imaging, while those with less elevated activity developed TTS several years later,” said Tawakol. “We show that TTS happens not only because one encounters a rare, dreadfully disturbing event—such as the death of a spouse or child, as the classical examples have it. Rather, individuals with high stress-related brain activity appear to be primed to develop TTS—and can develop the syndrome upon exposure to more common stressors, even a routine colonoscopy or a bone fracture.”
The process by which stress induces TTS is not well understood but may involve a multi-organ mechanism starting with activation of the stress-sensitive tissues of the brain. This brain activity in turn triggers several further events, including release of stress hormones, activation of the sympathetic nervous system and release of inflammatory cells, each of which can contribute to the development of TTS.
Limitations of the study include that it was a single-center, retrospective study that consisted mainly of patients with a diagnosis of cancer, a known TTS risk factor, which may limit how well the findings can be generalized. The researchers were unable to measure instantaneous changes in brain activity in response to a stressful event that led to TTS and so were not able to directly show a causal relationship. Nor were they able to measure changes in activity in other regions of the brain, which could also play a role.
Thinking about applying the results to the clinic, Tawakol said he hopes that interventions that lower stress-related brain activity will make it more difficult to develop TTS. “Studies should test whether such approaches to decrease stress-associated brain activity decrease the chance that TTS will recur among patients with prior episodes of TTS,” he commented, further noting the need for more studies into the impact of stress reduction—or drug interventions targeting stress-related brain activity—on heart health.



