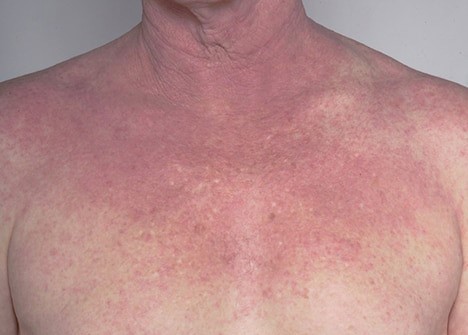
Aslan Pharmaceuticals said today it has acquired full global rights to develop, manufacture, and commercialize CSL’s atopic dermatitis candidate ASLAN004 in all indications, in a change to a five-year-old collaboration that could generate more than $780 million for CSL.
ASLAN004 is a fully human monoclonal antibody designed to target the IL-13 receptor α1 subunit, or IL-13Rα1. By targeting IL-13Rα1, ASLAN004 potently inhibits signaling of both interleukin 4 (IL-4) and interleukin 13 (IL-13), which are key to triggering symptoms of allergy in atopic dermatitis.
Aslan cited positive data announced March 29 following completion of the first part of a Phase I single ascending dose (SAD) study assessing the intravenous formulation of the ASLAN004 in healthy volunteers. The study (NCT03721263), conducted in a single Singapore site, included an analysis of phosphorylation of STAT6 (pSTAT6), a mediator of allergic inflammation that showed complete inhibition within one hour of dosing.
That inhibition “was then maintained for more than 29 days, suggesting monthly dosing may be achievable,” Aslan observed at the time.
The company also boasted that ASLAN004 was a more than competitive alternative to a competing drug, the marketed atopic dermatitis candidate, Sanofi/Regeneron Pharmaceuticals’ Dupixent® (dupilumab): “Although there are several drugs in development for atopic dermatitis, no-one has yet demonstrated once-monthly dosing with efficacy comparable or superior to dupilumab.”
“Best-in-class” potential
Aslan said it expects to report data from the second part of the study, testing a subcutaneous formulation, “shortly,” with plans to launch a multiple ascending dose study in moderate to severe atopic dermatitis patients in the second half of this year.
“We are very excited by the recent data we have generated on ASLAN004 and we believe that it has the potential to be a best-in-class treatment for atopic dermatitis and other inflammatory indications with a differentiated profile,” Aslan CEO Carl Firth, PhD, MBA, said today in a statement. “We look forward to reporting further data on ASLAN004 in atopic dermatitis and investigating its potential in other inflammatory indications.”
Aslan agreed to pay CSL $30 million upon the launch of a phase III study of ASLAN004, up to $95 million in payments tied to achieving regulatory milestones, up to $655 million tied to achieving sales milestones, as well as tiered royalties on net sales between mid-single digits and 10%.
Under the companies’ original agreement, signed in May 2014, Aslan agreed to oversee development of ASLAN004 (then known as CSL334) through proof-of-concept and identification of a partner to complete Phase III development and commercialization.
In return, CSL was eligible to receive between 40–50% of all ASLAN004 revenues, including proceeds from out-licensing agreements, Aslan said today; the companies initially did not disclose financial terms.
“An important achievement”
“The amendment of our agreement with CSL is an important achievement in our strategy to gain greater commercial control and retain more value from our pipeline programs,” Firth said.
On January 29, Aslan announced a corporate restructuring under which it designated ASLAN004 a co-lead product with acute myeloid leukemia (AML) candidate ASLAN003 and varlitinib, a candidate in biliary tract cancer (BTC).
The restructuring came two weeks after Aslan acknowledged the failure of varlitinib in a Phase II trial in another oncology indication, HER1/HER2-positive advanced/metastatic gastric cancer. In disclosing Phase II results on January 14, Aslan said patients treated with varlitinib plus mFOLFOX6 had an average tumor shrinkage of 22.0% after 12 weeks compared to 12.5% for patients treated with mFOLFOX6 alone, but added: “This difference did not reach statistical significance.”
As part of the restructuring, Aslan eliminated 30% of its workforce and cut costs, with the goal of reducing its operational expenses by 50%. The company had 56 full-time employees as of December 31, 2018, according to its Form 20-F annual report for last year, filed April 29. Of those employees, 28 were engaged in full-time research and development and 28 were engaged in full-time general and administrative functions.
Concurrent with the restructuring Aslan’s Chief Medical Officer Bertil Lindmark, MD, PhD, announced his retirement, and was succeeded on an acting basis by Chih-Yi Hsieh, MD, who was promoted from vice president, medical and general manager, Taiwan. Another Aslan executive, Mark McHale, PhD, COO, was shifted to chief development officer and head of R&D.