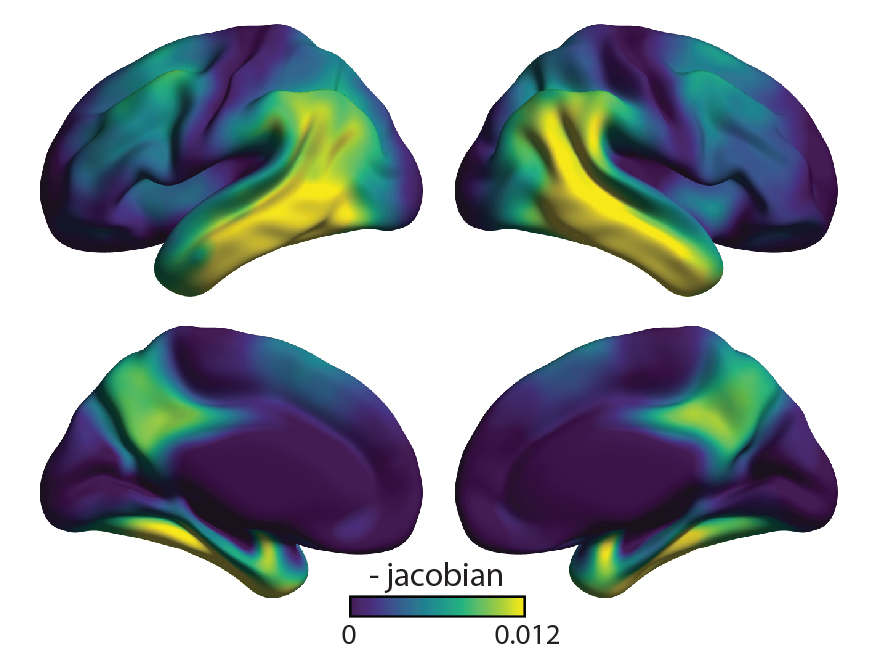
PET scans of patients with early Alzheimer’s disease showed that binding of a marker of tau protein correlated to regions of atrophy in the brain (colored areas). [R. La Joie et al., Science Translational Medicine (2019)]
The results of a prospective brain imaging study headed by scientists at the University of California, San Francisco (UCSF), support increasing evidence suggesting that tau pathology, rather than β-amyloid plaque formation, may be involved in driving brain degeneration in Alzheimer’s disease. The study, reported in Science Translational Medicine, demonstrated how the use of recently developed positron emission tomography (PET) tracer for visualizing and quantifying pathological tau protein tangles in the brains of Alzheimer’s disease (AD) patients could predict the location of future brain atrophy a year or more in advance. In contrast, PET imaging the location of β-amyloid (Aβ) plaques showed little utility in predicting how damage would increase as the disease progressed.
“The match between the spread of tau and what happened to the brain in the following year was really striking,” said neurologist Gil Rabinovici, MD, the Edward Fein and Pearl Landrith distinguished professor in memory and aging and leader of the PET imaging program at the UCSF Memory and Aging Center. “Tau PET imaging predicted not only how much atrophy we would see, but also where it would happen,” Rabinovici added. “These predictions were much more powerful than anything we’ve been able to do with other imaging tools, and add to evidence that tau is a major driver of the disease.” The scientists reported their findings in a paper titled, “Prospective longitudinal atrophy in Alzheimer’s disease correlates with intensity and topography of baseline tau-PET.”
Tau tangles and amyloid plaques are the two kinds of misfolded protein clusters seen in post-mortem brain samples from Alzheimer’s disease patients, and are thought to play a “crucial role in the neurodegenerative cascade that results in the loss of neurons and synapses,” the authors wrote. While researchers have long considered the relative importance of each type of abnormal protein deposition in the neuropathology of Alzheimer’s disease, over recent decades drug development has been skewed towards amyloid-targeting drugs, but with disappointing or mixed results.
Scientists are now reassessing whether tau protein—which was once dismissed as simply a “tombstone” marking dying cells—may represent a more important biological driver of the disease. In contrast to β-amyloid, which accumulate widely across the brain, sometimes even in people with no symptoms, autopsies of Alzheimer’s disease patients have shown that tau is concentrated precisely where brain atrophy is most severe, and in locations that help explain differences in patients’ symptoms (in language-related areas vs. memory-related regions, for example).
“No one doubts that amyloid plays a role in Alzheimer’s disease, but more and more tau findings are beginning to shift how people think about what is actually driving the disease,” explained Renaud La Joie, PhD, a postdoctoral researcher in Rabinovici’s in vivo molecular neuroimaging lab, and lead author of the new study. “Still, just looking at postmortem brain tissue, it has been hard to prove that tau tangles cause brain degeneration and not the other way around. One of our group’s key goals has been to develop noninvasive brain imaging tools that would let us see whether the location of tau buildup early in the disease predicts later brain degeneration.”
The development of PET radiotracers that bind either the β-amyloid plaques, or to misfolded tau in the brain has made it possible to investigate the role of both β-amyloid and tau in Alzheimer’s disease progression, by visualizing and quantifying AD pathology in living patients. “Those imaging biomarkers offer an opportunity to improve patient diagnosis and to study the development of AD pathophysiology by describing the relationships between protein aggregation, neurodegeneration, and cognitive impairment,” the authors wrote. Such studies increasingly suggest that it may be abnormal tau protein, rather than Aβ deposits, that is directly linked with cognitive decline and neurodegeneration in Alzheimer’s disease.
Rabinovici and collaborator William Jagust, MD, of UC Berkeley and Lawrence Berkeley National Laboratory, have been among the first to adopt tau-PET imaging to study the distribution of tau tangles in the normally aging brain, and in a smaller, cross-sectional study of Alzheimer’s disease patients. Their newly reported research represents the first attempt to test whether tau levels in Alzheimer’s disease patients can predict future brain degeneration. “Our primary hypothesis was that the tau deposition detected with FTP-PET drives, and therefore precedes, regional neurodegeneration in early symptomatic AD,” the authors wrote. “From a precision medicine perspective, we were interested in testing PET imaging’s ability to predict neuroimaging changes at the individual patient level.”
La Joie recruited 32 participants with early clinical-stage Alzheimer’s disease through the UCSF Memory and Aging Center. Each patient underwent PET scans using separate PET tracers to measure levels of amyloid protein and tau protein in their brains. The participants also underwent MRI scans to measure their brain’s structural integrity, both at the start of the study, and again in follow-up visits, one to two years later.
The researchers found that overall tau levels in participants’ brains at the start of the study predicted how much degeneration would occur by their follow up visit (which was on average 15 months later). The local patterns of tau build-up could also predict subsequent atrophy in the same locations with more than 40% accuracy. “The predictive value of the baseline tau-PET pattern on future atrophy remained substantial even after adjusting for baseline cortical thickness, with tau-PET explaining ~40% of unique variance in longitudinal atrophy,” the scientists wrote. In contrast, baseline amyloid-PET scans correctly predicted only 3% of future brain degeneration. Interestingly, the PET scans showed that younger study participants had higher overall levels of tau in their brains, as well as a stronger link between baseline tau and subsequent brain atrophy, compared to older participants. This indicates that other factors—likely other abnormal proteins or vascular injuries—may play a larger role in late-onset Alzheimer’s, the researchers further suggested.
“In line with our original hypotheses, we found that baseline tau PET, but not Aβ-PET, predicted the degree and spatial distribution of cortical atrophy over the subsequent year,” the investigators commented. “Together, these longitudinal results expand on previous findings from postmortem and cross-sectional studies, by providing prospective evidence that the aggregation of tau predicts future neurodegeneration in patients with biomarker-confirmed AD. These results support a sequential relationship between tau fibrillar aggregates and downstream degeneration.” La Joie added, “We demonstrated that tau-PET is not only predictive of how much but also of where atrophy will occur, which has major implications for patient prognosis and clinical trials. Seeing that tau build-up predicts where degeneration will occur supports our hypothesis that tau is a key driver of neurodegeneration in Alzheimer’s disease.”
The results support hopes that tau-targeting drugs, including those under investigation at the UCSF Memory and Aging center, may benefit patients by blocking this key driver of Alzheimer’s disease neurodegeneration. At the same time, the ability to use tau PET to predict later brain degeneration could enable more personalized care and speed clinical trials, the authors said. “One of the first things people want to know when they hear a diagnosis of Alzheimer’s disease is simply what the future holds for themselves or their loved ones. Will it be a long fading of memory, or a quick decline into dementia? How long will the patient be able to live independently? Will they lose the ability to speak or get around on their own? These are questions we can’t currently answer, except in the most general terms,” Rabinovici said. “Now, for the first time, this tool could let us give patients a sense of what to expect by revealing the biological process underlying their disease.”
Rabinovici’s team also anticipates that the ability to predict future brain atrophy based on tau PET imaging will allow Alzheimer’s clinical trials to quickly assess whether an experimental treatment can alter the specific trajectory predicted for an individual patient. This currently isn’t due to the wide variability in how the disease progresses from individual to individual. “Our findings suggest that tau-PET could be useful for the design of clinical trials and could increase the ability to detect a treatment effect even over a relatively short time frame,” they stated. “First, tau-PET could be used to enrich trials with patients with tau-PET signal predictive of upcoming atrophy or to stratify patients in trials based on the degree of expected atrophy in the upcoming year. Second, tau-PET could help determine how (i.e., where) atrophy should be measured to maximize study sensitivity.”
Such insights could make it possible to adjust dosage or switch to a different experimental compound if the first treatment is not affecting tau levels or altering a patient’s predicted trajectory of brain atrophy. “Tau PET could be an extremely valuable precision medicine tool for future clinical trials,” Rabinovici said. “The ability to sensitively track tau accumulation in living patients would for the first time let clinical researchers seek out treatments that can slow down or even prevent the specific pattern of brain atrophy predicted for each patient.”


