April 15, 2007 (Vol. 27, No. 8)
cAMP-Glo™ and Kinase-Glo® for Cost-effective Identification of Lead Modulators
GPCRs are involved in various physiological processes, such as the regulation of behavior, mood, and immune system activity. GPCRs represent one of the most important targets in drug discovery; approximately one-quarter of the best-selling drugs on the market today target GPCRs. Drug targets of such importance require well-designed assays that can speed up the discovery of novel drug candidates.
In order to sift through today’s complex compound libraries, drug developers need high-throughput assays that are easy to use, sensitive, precise, cost-effective, and able to accurately identify promising drug candidates quickly.
cAMP Measurement
This article highlights a system that combines acoustic dispensing, small-volume pipetting, and robust assay chemistries to identify GPCR modulators. This combination enables rapid screening of GPCRs in a 1,536-well format. Implementing this miniaturized, high-throughput GPCR assay allows cost-effective screening to identify lead modulators of GPCR signaling.
The Promega (www.promega.com) cAMP-Glo™ assay measures changes in cAMP levels, a hallmark of GPCR modulation. This homogeneous, bioluminescent assay is easily scalable to 1,536-well plate formats. The assay measures cAMP levels in cells by monitoring cAMP production in response to the effects of a test compound on GPCRs.
GPCRs that couple with adenylate cyclase will increase or decrease intracellular cAMP levels. The assay is based on the principle that cAMP stimulates protein kinase A (PKA) holoenzyme activity, decreasing available ATP and leading to decreased light production in a coupled luciferase reaction (Figure 1).
Binding of an extracellular ligand to its receptor alters the conformation of the associated heterotrimeric G protein (shown in orange and yellow in Figure 1), causing dissociation of the Ga and GbY subunits and initiating a cascade of cellular events. The alpha subunit, shown in yellow, is categorized into one of several groups: Gas, Gai/o, Gaq, and Ga12/13. Gas activates adenylate cyclase, while Gai/o inhibits adenylate cyclase activity.
As the concentration of cAMP increases, cAMP binds to PKA, supplied in the kit, and the regulatory subunits undergo a conformational change to release the catalytic subunits. The free catalytic subunits then catalyze the transfer of the terminal phosphate of ATP to a PKA substrate, consuming ATP in the process. The level of remaining ATP is determined using Kinase-Glo® (Promega), a luciferase-based-reagent. Luminescence is inversely proportional to cAMP levels. Thus, as cAMP concentration increases, luminescence decreases.
The cAMP-Glo assay can be performed in standard multiwell formats, including 96-, 384- or 1,536-well plates. Individual test compounds or compound libraries are transferred into the plates using an acoustic liquid handler (Echo® 555, Labcyte). Sound energy enables the dispensing of droplets of test compounds in 2.5-nL increments directly from source plates to the assay plates. No pipette tip or pin tool ever touches the compound samples during transfer. Additional advantages to using an acoustic liquid handler for dispensing compounds include precision (
After the compounds are dispensed into the assay plates, cells of interest are added. Adherent, suspension, or frozen cell cultures can be used with the cAMP-Glo Assay. Cells are treated with the test compound or compound library in an induction buffer containing broad-range phosphodiesterase inhibitors to inhibit cAMP hydrolysis for an appropriate period of time to modulate cAMP levels.
Cells and subsequent assay reagents can be dispensed into assay plates using a non-contact, liquid dispenser (Equator™ HTS, Deerac Fluidics) for accurate pipetting. Advantages of using the Equator include speed and accuracy, a 50 nL to 50 µL volume-dispense range, low dead volumes, and ease of use. Independent channel control can be used for dispensing gradients when optimizing assay conditions. Interchangeable deck components, including small- or large-volume capacity reservoirs, stirring devices, and multiple wash stations, or other customizable configurations, make this a flexible platform for high-throughput screening (HTS).
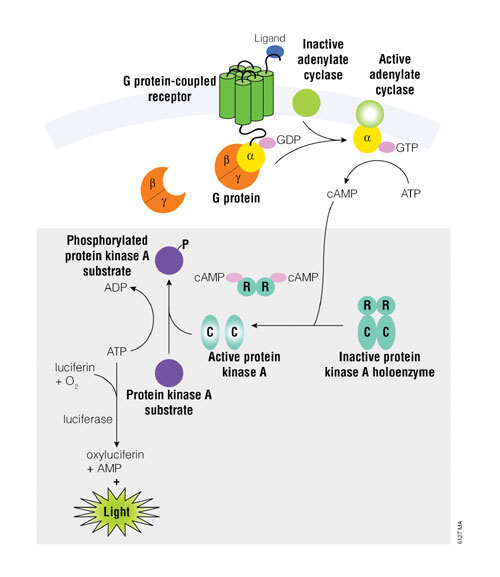
Figure 1
Detection
After induction, cells are lysed to release cAMP, followed by the addition of a cAMP-detection solution. This detection solution contains PKA and PKA substrate. The Kinase-Glo Reagent is subsequently added to terminate the PKA reaction and detect the remaining ATP via a luciferase reaction. Plates can then be read using a microplate-reading luminometer, or CCD-imaging device. Luminescence can be correlated to the cAMP concentrations by using a cAMP standard curve.
A notable feature of this assay is the speed and ease of reagent additions. Performance is not affected by the presence of up to 5% acetone or 5% DMSO, which are two common compound vehicles. Furthermore, because the half life for the luminescent signal is greater than four hours, this eliminates the need for luminometers with reagent injectors and allows batch-mode processing of multiple plates.
Titration experiments can be performed using the cAMP-Glo Assay to determine EC50, IC50, and Z´ Factor values for test or known compounds. That is, the protocol can be easily adapted to determine values of any agonist or antagonist in cells that express the target receptor for that agonist or antagonist, respectively. Data generated from these experiments can be used to select the optimal cell density or optimal compound concentration(s) for screening. All of this information can quickly be generated on a single assay plate.
Representative screening results with this HTS method are shown in Figure 2. In this study, 1-µL volume reactions were assembled in each well of a Corning 1,536-well plate. The d293 cell line containing the D1 receptor at a density of 1-K cells per well was treated with LOPAC1280 Library (Sigma-Aldrich) compounds and other known controls, each at a final concentration of 10 µM, with 1% final DMSO in the assay. Compounds were dispensed using the Labcyte Echo 555. Reactions were allowed to incubate at 37ºC for 30 minutes. Using the Equator HTS, all cAMP-Glo Assay reagents were added as follows: Lysis buffer (1 µL per well) was added to the treated cells, followed by a 30-minute incubation at room temperature; detection solution (2 µL per well) was added to the lysed cells, followed by a 20-minute incubation at room temperature; Kinase-Glo reagent (4 µL per well) was then added, followed by a ten-minute incubation at room temperature. Luminescence was recorded with a BMG Labtech PHERAstar plate reader, with a 0.25 second integration time per well.
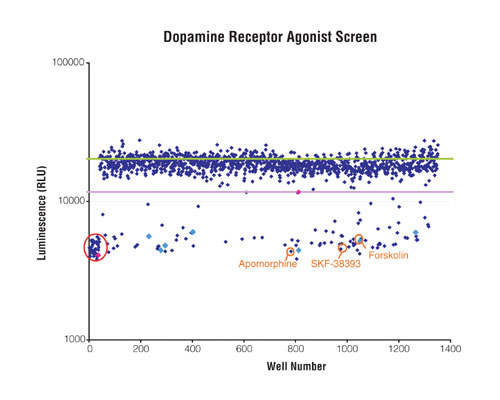
Figure 2
Results
In this miniaturized GPCR HTS study, 117 compounds were determined to be true hits. The majority of hits were compounds in the dopamine class, as expected based on the D1 d293 cell line selected. Secondary hits were found to be in the adrenoreceptor, serotonin, and cyclic nucleotide classes of compounds. Further HTS analyses of these hits would include titration experiments with the cAMP-Glo Assay to determine potency.
The combination of acoustic dispensing, small-volume pipetting, and a robust assay chemistry makes rapid HTS of GPCRs a possibility for any screening laboratory. Luminescent assays, such as the cAMP-Glo Assay described here, can be performed on a single 1,536-well plate, with only one endpoint readout needed to capture data. Performing a high-throughput screen on a single plate allows one to study hundreds of test compounds simultaneously, simplifying data analysis and sample tracking. Implementing this high-throughput miniaturized GPCR assay using the tools demonstrated in this article allows cost-effective screening to identify lead modulators of GPCR signaling.
Sarah Shultz and Tracy Worzella are automation scientists in the integrated solutions and engineering department at Promega.
Web: www.promega.com. Phone: (800) 356-9526.
E-mail: [email protected].


