Messenger RNA (mRNA) technology is beginning to show its immense potential in medicine. Indeed, according to Sudha Chivukula, PhD, head of discovery biology, mRNA Center of Excellence, Sanofi, mRNA technology is inaugurating a new era in the development of vaccines and therapeutics.
“A true testimony for this technology will be revealed in the coming years as more and more clinical data from vaccine and therapeutic applications are made available,” Chivukula declares. “Any infectious disease that can be prevented by a protein is a potential application for mRNA vaccines.” He adds that mRNA technology has the potential to transform rare disease medicine, oncology, and personalized medicine.
Already, mRNA-based vaccine and therapeutic candidates are proliferating. Which ones should be prioritized? To answer that question, developers must weigh various factors. The classic factors include potency, safety, and stability. With mRNA-based vaccines and therapeutics, however, there may be additional considerations. For example, delivery mechanisms may matter as much as mRNA sequences. Also, different mRNA platforms may vary with respect to flexibility (switching from one target to another, or even hitting multiple targets) and manufacturability (altering some processing steps while preserving others).
Weighing mRNA platform considerations
To narrow down the number of candidates to be evaluated in preclinical trials, developers can employ high-throughput automated robotic platforms. “Innate and adaptive responses to mRNA vaccines in animal models help to interrogate the immunological mechanism that underlie efficacy and reactogenicity,” Chivukula elaborates. “New translational tools based on in vitro human immune system models could be deployed to better predict clinical outcomes.”
Most common in pediatric vaccination, combination vaccines have successfully controlled several infectious diseases by combining antigens from separate and defined manufacturing process. While this adds complexity, it also creates opportunities to integrate both viral and bacterial antigens into a single vaccine dose.
Ideally, mRNA-compatible manufacturing platforms should be available, versatile, and broadly applicable, allowing for a simple swapping of the target gene sequence while largely maintaining the rest of the process. Such platforms accelerate vaccine development.
The “extras” that come with mRNA technologies are not limited to the flexibility and manufacturability issues considered thus far. Another extra is in the realm of immunity. Besides providing the target genes to train the immune system, mRNA is intrinsically immunomodulatory. Consequently, using mRNA can lead to augmented immune responses.
“mRNA vaccines mimic infection or immunization with live microorganisms and stimulate potent T follicular helper cell responses and germinal center B cell responses leading to generation of protective antibodies,” Chivukula continues. “mRNA packaged in lipid nanoparticles can produce complex antigens in vivo, via engagement of endogenous ribosomal machinery, with proper post-translational modifications including assembly. All the mRNA requires is delivery into the cytosol for translation. No risk of host genome integration is incurred.”
With mRNA technology, it is also possible to adopt in silico design approaches that could facilitate the development of multivalent vaccines. Such vaccines could be particularly effective against influenza.
“We recognize that addressing the broader consequences of influenza virus infection—such as severity of disease, extent of morbidity and mortality—is a challenge for any novel technology,” Chivukula notes. “While it is a high bar to further improve our differentiated vaccines, we hope mRNA platform technology will help improve the effectiveness of vaccines against this persistent respiratory infectious disease.”
Chivukula also observes that in silico design approaches could expedite vaccine development, helping developers “deliver high volumes on short timeframes.” In addition to countering multiple strains, new vaccines for upcoming influenza seasons could be manufactured more expeditiously.
Changing the message
“With an mRNA platform, the only thing that changes is the ‘m,’ the message,” says Brad Sorenson, president and CEO, Providence Therapeutics. “Our strengths are both in designing the mRNA and manufacturing it at high purity.”
Founded in 2015, Alberta-based Providence focuses on identifying immunological targets and developing personalized cancer vaccines for glioblastoma, ovarian cancer, and breast cancer. Providence was set to begin clinical trials with a lead candidate in 2020, but then a different vaccine crisis arose, prompting the company to begin applying its technical expertise in mRNA to a SARS-CoV-2 vaccine.
“Our next step for our SARS-CoV-2 vaccine is to run a Phase III booster trial to address the endemic nature of the virus,” Sorenson states. “Since there are now existing vaccines, emergency use authorization for approval is unlikely, and this has the effect of lengthening the path for regulatory approval. Based on this, we expect approval in early 2023.”
The unstable mRNA needs protection during delivery, and lipid nanoparticles are typically used to encapsulate it for cellular delivery. To provide such protection, Providence started developing its own delivery technology. However, this technology would not have been ready for use with the company’s COVID-19 vaccine, which needed to be developed extraordinarily quickly. So, delivery technology was licensed from Genevant Sciences. “Genevant’s suite of technology showed that it worked for intramuscular (IM) injection,” Sorenson notes.
Besides working on a vaccine for the parental SARS-CoV-2 strain, Providence is working on a vaccine for the Omicron variant as well as a long-term vaccine with a different mechanism to cover a wider class of coronaviruses. The long-term vaccine would also address variants. Preclinical work looks promising.
Providence is working with China-based Everest Medicines
on COVID-19 vaccines. “We are looking for additional like-minded partners for full technology transfer to ensure our vaccine gets deployed globally,” Sorenson remarks. “We also want to see more targets explored in general. Great academic scientists have identified targets and want access to mRNA platforms. We collaborate with them or support their research to design and manufacture the mRNA.”
Harnessing epigenetics
“Epigenetics is nature’s mechanism to control gene expression and cellular growth,” says Mahesh Karande, president and CEO, Omega Therapeutics. “All of our genes along with their regulatory elements are located in loops of DNA called insulated genomic domains (IGDs).” He adds that at the base of each IGD loop there are CTCF proteins that insulate the IGD from “outside transcriptional machinery.”
There are about 15,000 IGDs, and they act as fundamental regulators of the human genome. IGDs are ubiquitous in every cell, and they are distributed across the 23 chromosomes. “This is nature’s operating system,” Karande notes. “[It] has enabled us to create the OMEGA Epigenomic Programming Platform.”
All the regulatory elements within an IGD, such as promoters, enhancers, and CTCF locations, have unique sequences that Omega refers to as epigenomic zip codes, or EpiZips. They can be used as drug targets to control gene expression. Although CTCF sequences are conserved evolutionarily, their flanking sequences in each IGD are unique. “All of the EpiZips that control single or multiple genes within an IGD can be targeted with high specificity, enabling the control of gene expression,” Karande asserts.
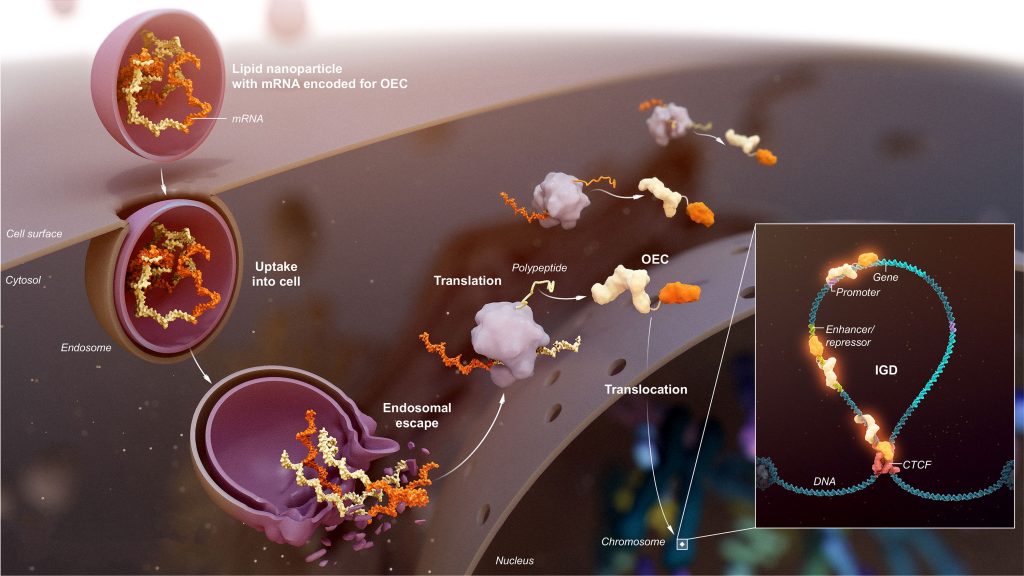
Omega’s epigenomic programming platform contains a proprietary database of thousands of EpiZips. To target the EpiZips, Omega deploys its aptly named Omega Epigenomic Controllers (OECs), which are modular and programmable mRNA medicines. OECs include a DNA-binding domain to home in on and bind to a specific EpiZip and an effector domain, which varies depending on the modulating effect required.
“We can target a gene with high specificity and tune it to the right level of expression with programmable durability to treat acute and chronic diseases,” Karande explains. “We are not changing native DNA sequences but rather tuning the malfunctioning expression system and restoring it to normal levels. The approach is personalized to the disease, not the person.”
An IND filing is expected this year for a therapeutic that addresses hepatocellular carcinoma by regulating overexpression of the highly autoregulated (and heretofore undruggable) MYC gene. “The beauty of our platform is that it is a deterministic, prospective, and programmable platform,” Karande emphasizes. “Our ability to modulate disease is extremely broad.
“We tune genes pre-transcriptionally for controlled disease-specific duration. The specificity of targeting and the fact that our drug does not have to be continuously resident to have effect is why we believe our approach will lead to safer and more efficacious therapeutics than current modalities.”
Treating the brain
RNA-based therapeutics against neurodegenerative diseases are being developed by Biorchestra, which bases its target discovery work on the research performed by Branden Ryu, PhD, the company’s founder and CEO. Ryu discovered a novel brain regulator of inflammation, MiR-485-3p, that plays a role in common, neurodegenerative disorders such as Alzheimer’s disease. To downregulate MiR-485-3p, the company developed an antisense oligonucleotide (ASO) as well as a novel nanoparticle for delivery of the ASO to the brain.
The highly specific ASO is a relatively small molecule compared with other forms of RNA or RNA derivatives. “By combining designs of both the chemical elements and the bioinformatics elements, and by looking for potential overlap with known sequences, you significantly pare down the risks of off-target effects,” says Louis O’Dea, MB, BCh, BAO, the chief medical officer of Biorchestra and the president of the company’s North American subsidiary.
With central nervous system therapeutics, the Holy Grail is getting the drug to the brain. Some small-molecule drugs pass into the brain quite readily, but larger molecules like antibodies cannot. Although lipid nanoparticles are suitable for liver uptake, as they target the cholesterol receptor on liver cells, they have no affinity for the brain.
“We wanted to extend the lipid nanoparticle approach that facilitates the liver’s uptake of RNA-based drugs,” O’Dea relates. “Our goal was to develop a delivery system uniquely focused on facilitating the brain’s uptake of RNA-based drugs.
“Certain proteins are preferentially absorbed by the brain for its own benefits,” O’Dea adds. “That understanding allowed us to attach a ligand to our nanoparticle that is recognized by the cells regulating entry into the brain. This ligand coating of the nanoparticle allows about 60-fold more of the drug to enter the brain.
“Our approach provides the potential to treat Alzheimer’s disease. MiR-485-3p is also overactive in Parkinson’s disease and amyotrophic lateral sclerosis. We have shown cognitive improvement in animal models of Alzheimer’s disease, as well as functional improvement in animal models of Parkinson’s disease and amyotrophic lateral sclerosis.”
The overexpression of MiR-485-3p occurs in about 90% of Alzheimer’s patients, leading to inflammation and beta-amyloid and tau protein overproduction. Biorchestra is not directly targeting these proteins but rather the process that causes their overexpression. The company’s approach targets upstream processes and thereby reduces and clears both beta-amyloid and tau protein.
“Our central nervous system delivery platform can facilitate the work of other companies,” O’Dea asserts. “We are open to discussing strategic partnerships to help patients access life-changing therapeutics more quickly.”
Improving delivery
According to Anders Høgset, PhD, chief scientific officer, PCI Biotech, it is well known that problems with endosomal release represent a major barrier for the delivery of therapeutics, especially nucleic acid therapeutics. Even with the best systems used today (for example, lipid nanoparticles), only a few percent of the nucleic acid cargo will be released from the endocytic vesicles.
The company’s photochemical internalization (PCI) technology enables the light-triggered release of molecules from endosomes. It has been used to enable the release of up to 50% of an endocytosed protein.
“Since the PCI endosomal release is triggered by illumination, delivery with PCI can be strictly regulated both in space and time,” Høgset explains. “Delivery will only be induced in illuminated areas of the body after illumination occurs. PCI achieves targeted delivery of therapeutic molecules.”
The photosensitizing molecule, fimaporfin, is designed to accumulate specifically in the membrane of endocytic vesicles. When illuminated, the fimaporfin molecule will take up energy from the light and be excited into a high-energy, unstable state.
The energy from this molecule’s unstable state is then transferred to other molecules, most importantly to oxygen, to generate reactive oxygen species (ROS). The ROS will react with other molecules in the endosomal membrane, leading to membrane permeabilization so that the contents of the endocytic vesicles will leak into the cytosol. “Importantly,” Høgset states, “illumination from a suitable light source is directed only at the site that the PCI effect is desired.”
PCI Biotech’s fimaVacc technology is used for immunotherapeutic purposes, especially to deliver antigens into the cytosol of antigen-presenting cells to increase MCH Class I antigen presentation and enhance cytotoxic T-cell responses. The antigen can be delivered in the form of peptides, proteins, or antigen-encoding mRNA molecules.
The company’s fimaNAc technology is used to deliver various types of nucleic acids in all other therapeutic areas, such as regenerative medicine and treatment of various skin conditions. “The therapeutic aim of fimaVacc and fimaNAc is different,” Høgset emphasizes. “Overall, PCI can increase the efficacy of nucleic acids, reduce their off-target effects, and achieve targeted, local delivery of therapeutics. We are open to collaboration in the nucleic acid therapeutic space.”



