Associate Director, Product Management, Personalis
Liquid biopsies are increasingly recognized as a valuable tool in oncology. Noninvasive and relatively inexpensive, they identify circulating tumor DNA (ctDNA) in the blood and can be used longitudinally over the lifetime of a disease to manage its treatment and monitor its recurrence. And the applications of liquid biopsies continue to expand. Through advances in whole-genome sequencing (WGS), the tumor-informed liquid biopsy is becoming a key tool in oncology drug development.
Sensitivity barriers
Some liquid biopsies are tumor-agnostic, which means that they test a fixed panel of the most common tumor mutations or epigenetic markers without incorporating an individual’s tumor variant profile. While these tumor-agnostic liquid biopsies can deliver faster results, the lack of tumor-specific information—and thus sensitivity—can limit their utility.
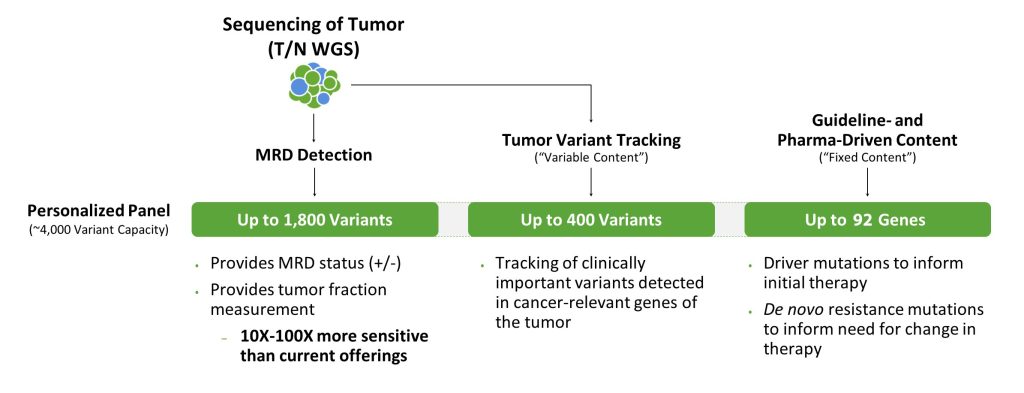
Comparatively, tumor-informed liquid biopsies are significantly more sensitive. In this case, an individual’s tumor is first sequenced to identify specific mutations, which are then used to design a personalized panel. Tumor-informed liquid biopsies test and track those specific mutations, which can enable detection of tumors in earlier stages and longitudinally post-diagnosis.
But there are significant differences between tumor-informed liquid biopsies as well. Most developers of liquid biopsies use exomes to derive tumor mutations from the tissue and inform personalized panels. While these can be more sensitive than conventional tests used to detect tumors, such as CT scans, they may not have the sensitivity necessary to detect cancer at earlier stages when there is a greater potential to treat and prevent cancer from metastasizing. This is because assay sensitivity is driven by the number of mutations in the personalized panel. Exomes do not typically have a lot of variants in them, limiting the ability to create a large enough panel to achieve the needed sensitivity.
WGS of tumor tissue DNA
Personalis presented a poster at the 2022 annual meeting of the American Association for Cancer Research showing that liquid biopsies informed by WGS of tumor tissue DNA enable new levels of performance via ultrasensitive panels.1
To maximize the chances of detecting cancer earlier, the goal is to collect as many mutations from a solid tumor as possible. WGS of tumor tissue DNA provides information on many mutations because it examines the entire genome; an exome represents only 1–2% of the genome. For tumors that traditionally have low mutational burdens, such as those from breast cancer, WGS produces roughly 6,000 mutations, whereas exome sequencing produces only 60 mutations. For tumors with high mutational burdens, WGS sequencing can provide hundreds of thousands of mutations.
Also, WGS reaches noncoding regions that often contain a rich collection of high-frequency, low-noise variants that best inform the development of highly sensitive, customized liquid biopsy panels. These variants are missed by exome or panel-based approaches.
Drug development implications
Drug development is costly, time-consuming, and risky, with a historically high failure rate. For those that succeed, it costs $1.3 billion to bring a new drug to market.2 Highly sensitive liquid biopsies informed by WGS of tumor tissue DNA provide pharmaceutical companies an opportunity to increase drug development success, whether they are embarking on developing an entirely new drug therapy or repurposing an approved drug.
Traditionally, the molecular residual disease (MRD) status of patients is unknown during trial enrollment. A key application of tumor-informed liquid biopsies based on WGS is to ensure that patient cohorts are MRD positive. With this knowledge, drug developers can screen fewer participants (compared to using less-sensitive MRD assays), reduce trial sizes, and have the confidence that patient cohorts are composed of individuals who have the potential to be most impacted by the investigational drug.
For example, in a retrospective analysis, IMvigor trial (resectable urothelial carcinoma) investigators found that MRD-positive patients given adjuvant atezolizumab had better disease-free survival and overall survival versus MRD-positive patients in the observational arm.3 There was no significant difference in either metric in either arm for those who measured MRD negative.
In addition, because tumor-informed liquid biopsies based on WGS can be used longitudinally throughout trials, developers have the ability to monitor the impact of drug therapy in real time. This enables developers to more closely measure therapeutic response and optimize care, as well as to mitigate false negatives caused by the ultrasensitivity of liquid biopsies. Recent data from the PADA-1 breast cancer trial saw disease-free survival double upon switching from an aromatase inhibitor to fulvestrant based on the emergence of an ESR1 mutation.4
These applications have the potential to notably reduce the length and cost of drug development by helping to identify the surrogate endpoints of clinical trials. By using ultrasensitive liquid biopsies based on WGS, drug developers can accurately measure ctDNA levels in blood much earlier than by using conventional tests. With earlier indications of ctDNA clearance—when patients no longer test MRD positive—drug developers could reduce ongoing trials by months or years, resulting in significant economic savings and earlier submissions to the FDA.
Dan Norton is associate director of product management at Personalis.
References
- Boyle et al. A high sensitivity, tumor-informed liquid biopsy platform, designed to detect minimal residual disease at part per million resolution; Poster presented at: 2022 Annual Meeting of the American Association for Cancer Research; April 8, 2022; New Orleans, LA.
- Wouters et al. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009–2018. JAMA 2020; 323(9): 844–853.
- Rouprêt M, Gschwend JE. Overall survival (OS) by circulating tumor DNA (ctDNA) status in patients with post-operative muscle-invasive urothelial carcinoma (MIUC) treated with atezolizumab (atezo): Update from IMvigor010. Proceedings of the 2022 European Association of Urology Annual Congress. July 4, 2022; Amsterdam.
- Bidard et al. Fulvestrant-palbociclib vs. continuing AI-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2–metastatic breast cancer patients: Results of PADA-1, a UCBG-GINECO randomized phase 3 trial. Proceedings of the 2021 San Antonio Breast Cancer Symposium. December 9, 2021. San Antonio, TX.