February 1, 2011 (Vol. 31, No. 3)
Cell-Line Optimization Takes Many Forms, and Each Approach Has Its Adherents
While cell-service companies may argue persuasively for their pet technologies, cell-line optimization can proceed from several different angles.
Andrew Sandford, vp of business development at Selexis, describes his company as a “pure-play developer of technologies for rapidly producing stable, high-performing cell lines for bioproduction.”
The company’s rapid cell-line development technology, Selexis Genetic Elements™ (SGEs), are human DNA sequences that control the dynamic organization of transgenes in the chromatin. SGEs insulate nearby genes from the effect of surrounding chromatin, thereby increasing copy-number-dependent, position-independent gene expression. From a cell-line development perspective, SGEs increase the number of independently transformed cells that express the protein of interest, and promote higher levels of protein expression.
SGEs, which are found only in higher organisms, also possess regulatory functions that control the expression of many genes at a time. “There are thousands of these elements in the genome,” says Pierre-Alain Girod, Ph.D., CSO. Not all elements work in every cell line or species. Selexis has tested SGEs that are optimal for CHO, HEK (human embryonic kidney), CAP (Cevec amniocyte production), and other biomanufacturing-worthy cells.
“SGEs boost not only the number of copies of DNA integrated into the genomes of mammalian cells, but the quantity of messenger RNA generated, and makes the integration sites more stable to long-term expression,” Sandford explains. “Because of how these elements work, we can condense development time to 10 to 12 weeks post-transfection, from gene to a stable clonal production cell line.” Selexis claims titers of between three and five g/L for optimal Chinese hamster ovary (CHO) cell lines, and between one and three g/L for their “bread and butter” unoptimized batch processes.
In addition to its cell-line development and gene-expression platforms, Selexis employs a cell culture platform based on high-performing CHO cells that thrive in basal media and a specially designed feed regimen. Cell development and culture components must be synchronized, Sandford says, to achieve highly stable, productive cell lines in three months.
After transfection, Selexis uses antibiotics to kill off cells that do not carry the desired gene and limits dilution cloning to isolate cells. “Instead of trying to find a needle in a haystack, we have a stack of needles to choose from,” Sandford explains. “Not needing to engage in mechanical separation to isolate high-performing cells is a tremendous advantage from the perspective of throughput. We’re not limited by the number of flow cytometry systems we have.”
Lonza creates high-producing cell lines and processes for cells developed by customers as part of its comprehensive biopharmaceutical development services. Lonza’s cell-related services include vector construction, cell-line construction (transfection and cloning), cell-line evaluation in shake-flasks and bioreactors, cell bank creation and characterization, bioreactor process optimization, and bioreactor process characterization.
Lonza recently introduced a new cell-line construction process that transfects and clones in the same step. The process, which involves direct cloning from transfectant pools using fluorescence-activated cell sorting (FACS), reduces timelines from transfection to cGMP master cell bank creation to about 19 weeks. “As part of this construction, we use a screening process that is relevant to the production process,” says Alison Porter, Ph.D., senior science leader at Lonza’s Slough, U.K. facility. “The screens we use in cell-line creation are therefore designed to partner with our production process.”
The company’s philosophy regarding production is to develop platform inoculum and processes that employ standardized media, feeds, and culture conditions with model cell line(s). These provide speed to clinic while retaining the possibility of further optimization.
Since 1990 Lonza says its product titers have increased 200-fold as a result of improvements in specific production rate, viable cell concentration, and culture time. “Expression system, vector design and selection, and screening all contribute to this,” Dr. Porter notes, “but the major influencers of product concentration have been process conditions and the cell line, and of the former the design of media, and feeds in particular, have been of most importance.”
Moving forward, she believes that host cell-line engineering will likely result in further advances in product concentration, for example by controlling apoptosis and/or assisting translation and product secretion.

Lonza’s cell-related services include vector construction, cell-line construction (transfection and cloning), cell-line evaluation in shake-flasks and bioreactors, cell bank creation and characterization, bioreactor process optimization, and bioreactor process characterization.
Conserving Resources
Like most large companies with multiple pipeline and marketed biologicals, Genentech employs platform CHO cells to produce most of its materials for toxicology and early clinical trials. Optimization occurs only for projects that have passed developmental milestones. “Since we do not know the long-term success of any individual project, we minimize our investment in the early phases and avoid optimization unless either production levels are insufficient or undesirable product quality attributes are detected,” says John Joly, Ph.D., a scientist at the company. Cells targeted for additional development are typically about to enter Phase III studies. Cells and processes emerging from these efforts are carried through for commercial supply.
“A highly productive cell culture process depends greatly on the cell line, media, and process,” Dr. Joly notes. “This means starting with a host cell line that is highly conducive to generating high-yielding cell lines, screening to distinguish good from poor producers, designing experiments that will accurately reflect success at larger scale. Predictive scale-down models of our bioreactors early in the screening process has led to many successful cell culture processes at Genentech.”
Dr. Joly’s group relies on gene transfection and amplification techniques to produce high-yielding cell lines, clone-screening to select the best-performing lines, and media development to understand the impact of nutrients on yield improvement and product quality.
“We include design of experiment (DOE) for much of our work, whether it’s done at high, moderate, or low throughput. Given the rich history of producing biopharmaceuticals from CHO cells at Genentech, we are able to build upon what we learned during past projects.”
While Genentech hedges its bets during early development, the possibility that these cells may one day be called upon for full-scale production is always within view. Its scientists closely examine early-stage productivity, compare it with anticipated future demands, and determine if changes will be needed for either the cell line or process. “We then make rational choices about how much effort in cell line and process development are warranted.”
Ultimate Downstream Biomarkers
Numerous points of investigation, or intervention, are possible during cell-line development. DNA, RNA, and proteins are traditional places where developers look. Increasingly they also consider the metabolome—the t300–400 significant small molecule metabolites representing the downstream products of cellular activity.
Metabolon provides fee-for-service around its core technology, which uses conventional analysis tools (HPLC and mass spectrometry) to profile all small molecules in a sample. Metabolomics has been a hot research area in biology and medicine; now it is proving its mettle as a service to support biomanufacturing as well.
Metabolon conducted its first such study three years ago. In 2010 bioprocess-related work accounted for one-fourth of the firm’s 320 projects, says Mike Milburn, Ph.D., CSO.
“Our bioprocessing-related work focuses on helping companies pick which cell lines to investigate further,” Dr. Milburn says. Selecting the right cells, or rejecting the wrong ones, has a profound influence on manufacturing, bioprocessing, and product quality. Metabolomics, according to Dr. Milburn, provides greater insight into the “black box” of production cells, and affords bioprocessors an alternative to the “pure numbers game of high-throughput screening of culture conditions and many, many clones.”
A typical metabolomic project profiles all relevant metabolites and their concentrations, both inside cells and in the culture medium, over the course of a cell culture run. Intracellular analysis provides a “fingerprint” of cellular activity, and might lead to hypotheses about pathway activation or inactivation, either of which may be beneficial or detrimental to the process or affect product quality. These ideas are then confirmed (or not) by fingerprinting the medium for the extracellular counterparts of the same metabolites, as well as others. “We look for compounds in the medium that are limiting, which if added back might improve the process,” says Dr. Milburn.
Alternatively, one could identify a product outside the cell that suggests that key nutrients are being shunted to a suboptimal metabolic pathway. For example, one recent study showed that cells were producing too much sorbitol, a metabolite indicating that glucose is undergoing suboptimal metabolism. Sorbitol is expressed at high levels, for example, in cells undergoing apoptosis. “This was a clear indication that these cells were not as metabolically healthy in this medium as they should have been. In this situation, the study indicated clones that the sponsor should probably have avoided.”
Using metabolic biomarkers of cell suitability has other advantages as well. During early development cells are usually grown in rich media, where they appear to thrive and produce. “But that doesn’t mean the cell is healthy, or will be as robust in production media. The right biomarker can help processors triage out unhealthy cells very early on.”
Unlike genomic or proteomic cell line analysis, metabolomics is rapid and easily testable. Developers can add or remove ingredients, or change conditions, and test the changes over the course of several days. “It’s much more difficult, after making a genetic change, to know if it will directly affect the process or product.”
This is one reason why media and feed companies are so interested in metabolomics. As processing evolves toward more chemically defined media, the study of small molecules becomes indispensible for replacing vital nutrients and eliminating those that do not contribute to productivity.
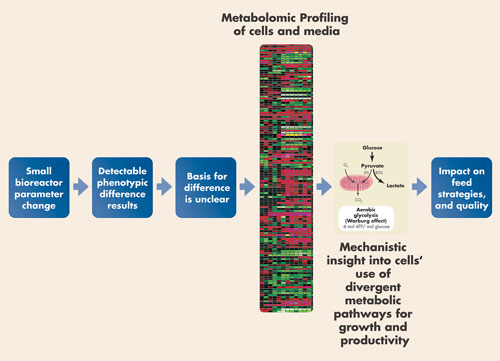
Metabolon interprets cellular metabolism changes to enhance process optimization from clone selection to manufacturing.
Nurture Becomes Nature
Sometimes lost in the nature-vs.-nurture debate on factors affecting productivity is the affect of process conditions on gene expression. For example, adapting attachment-dependent cells to suspension conditions can dramatically influence gene expression.
Using genome-wide expression profiles for CHO cells, Yung-Shyeng Tsao, Ph.D., senior principal scientist at Merck, identified more than 500 genes, and several major pathways that are “profoundly affected by suspension adaptation.”
As expected, pathways for cell-cell adhesion were downregulated, as were mechanisms for cell cycle, nucleotide synthesis, transcription, translation, and others. Upregulated pathways included those governing extracellular matrix, basement membrane, hypoxia signaling, and amino acid transporters. Genes controlling lipid transport and synthesis were upregulated by as much as a factor of 500.
Dr. Tsao’s principal approach to analyzing genomes is a CHO DNA microarray. Now in its third generation, the microarray was originally developed through the CHO Consortium, an academic-industrial group dedicated to improving CHO production cells. The microarray analyzes hamster ovary cells and other tissues as well. “CHO cells have been passaged for many generations, in many different locations, which has been selective for the expression or downregulation of certain genes,” Dr. Tsao explains. “So if you only examine CHO cells you cannot cover the entire genome.”
Merck is also profiling cell metabolism, partly in collaboration with Metabolon. This detailed analysis is too expensive and time-consuming to conduct for every project, but it does provide key insights into the ultimate status of cells. For select projects Dr. Tsao compares the genome, proteome, and metabolome. The overlaps as one moves downstream from genes to small molecules can confirm, for example, which genes ultimately affect cell performance.


