Carlos Obejero-Paz M.D., Ph.D. Principal Scientist ChanTest Discovery Services, Charles River
CiPA Initiative Aims to Better Detect Drug-Induced Cardiac Arrhythmias
With cardiovascular side effects an ever-pressing concern in drug safety, interest is growing in ion channel assays and in silico modeling, two methods that are part of the Comprehensive in vitro Proarrhythmia Assay (CiPA), a new initiative driven by FDA and industry efforts to better detect life threatening drug-induced cardiac arrhythmias.
The CiPA initiative came about after the realization that the current cardiac toxicity testing paradigm is too conservative, keeping potentially beneficial drugs off the market and, simultaneously, from patients who need them. At the same time, current methods are relatively poor predictors of drug-induced cardiac arrhythmias.
Current Cardiac Toxicity Testing
Drugs that prolong the QT interval of the electrocardiogram (ECG), the time between electrical excitation and recovery (repolarization) of the ventricles, show an increased likelihood of Torsades de pointes (TdP), a self-limited ventricular tachycardia (rapid heartbeat) that can evolve to a rapid and irregular heartbeat and sudden death.
This is obviously not a desired outcome, so to gauge the risk of drug compounds, regulatory authorities and the industry have been using a set of surrogate markers of TdP. The markers include an in vitro assay that measures inhibition of a cardiac channel encoded by the human ether-a-go-go related gene (hERG) and an in vivo ECG assay that measures prolongation of the QT interval in animals and humans.
The hERG assay is based on the fact that most cases of TdP occur in drugs that block this channel. As opposed to other potassium channels, the pore of the hERG channel can accommodate drugs from different classes, including bulky compounds, making the channel susceptible to blocking by both cardiac and non-cardiac drugs. The hERG channel underlies the rapid component of the delayed rectifier potassium current (IKr), a major repolarizing current in the heart. Thus, hERG channel block results in delayed repolarization of the cardiac action potential (measured as prolongation of the QT interval) and, in some circumstances, reactivation of depolarizing currents and proarrhythmic events.
But the hERG assay also has a flip side. While it undoubtedly weeds out unsafe drugs before they reach the market, the current regulation has also had an outsized impact on new drug development; about 60% of new molecular entities show hERG liability and are abandoned in early development.
This can be problematic for drug developers as not all hERG blockers are associated with increased TdP risk (verapamil, ranolazine) due to multiple ion channel effects (MICE).1 Moreover, prolongation of the QT interval is associated with drugs that block other potassium channels (JNJ 303) or activate depolarizing currents (alfuzosin).2,3 Overall, the QT assay is highly sensitive but not specific for predicting TdP risk.
A New Screening Approach
CiPA is a novel safety screening proposal that consists of two in vitro biological assays and one in silico computational analysis. The new paradigm, announced about two years ago, includes other major cardiac ion channels (as well as hERG) and integrates MICE using mathematical models of the human ventricular action potential. In silico simulations are compared with the effects on action potentials measured in stem cell-derived cardiomyocytes. The CiPA results, in conjunction with the Phase I ECG evaluation, would then be used to determine TdP liability.
The CiPA initiative is possible because of the mechanistic understanding of how ion channels, transporters, biochemical processes and changes in intracellular ion concentrations cause proarrhythmic events. Two images below illustrate this nicely. Figure 1 shows the components of the O'Hara-Rudy model of the adult human cardiomyocyte used in the CiPA initiative. The model consists of 41 differential equations that are solved to calculate changes in membrane potential and intracellular ion concentrations.4
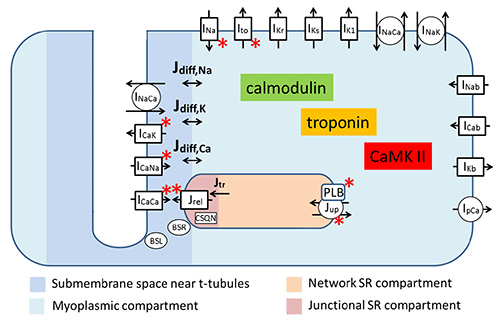
Figure 1. Physical interpretation of the human ventricular cardiomyocyte model modified from reference 4. Acronyms starting with I and J indicate ion currents and ion fluxes respectively. Note that the model addresses the presence of subcellular compartments.
The simulation in Figure 2 investigates the effect of increased hERG block. Note the presence of voltage oscillations named early after depolarizations (EADs) at ~91% hERG channel block. In a background of drug-induced or structural enhanced dispersion of repolarization, EADs can trigger TdP. The figure also shows an example of MICE where a 50% block of the calcium channel prevents the EADs induced by 91% hERG block. This simulation explains why drugs that block hERG and calcium channels prolong the QT interval but lack TdP risk.
These simulations were based on the “conductance block” model where currents in the presence of the drug are scaled by a factor proportional to the steady state current block. However, more complex models of drug-channel interaction are being evaluated at the FDA with the help of scientists from academia and industry including Charles River (Fermini et al., 2015, in press). The goal is to modify the O'Hara-Rudy model to evaluate ion channel pharmacology based on the kinetics of drug binding and changes in channel gating.
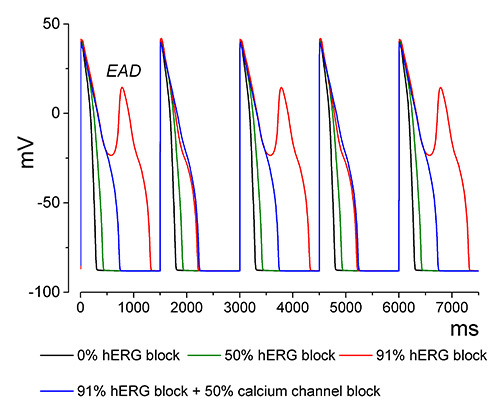
Figure 2. Steady state simulation of hERG and calcium channel block. The figure shows the last 5 of 1,000 action potentials paced at 0.67 Hz to steady state from four simulations with different levels of hERG and Cav1.2 block indicated in the figure.
The End Game
The final goal of CiPA is to obtain a scoring system for proarrhythmia based on the propensity to induce EADs and other mechanisms of repolarization instability. Toward this end, a training set consisting of high, intermediate and very low, or no TdP risk was established to evaluate drugs using manual voltage patch clamp. Because the industry commonly uses high-throughput voltage clamp platforms to assess cardiac ion channel effects, it will be necessary to compare both voltage clamp methodologies.
Will It Work?
An Oxford University study that used manual patch clamp to simulate changes in the action potential duration concluded that this is a better approach than considering hERG block alone.5 And at last year's Safety Pharmacology Society Meeting, we reported that changes in simulated action potential duration based on data obtained using an automatic voltage clamp platform are better predictors of TdP than hERG block alone.
As the field moves closer to the implementation of CiPA, more will be uncovered as to how to best apply this new methodology. ChanTest, a Charles River company, along with colleagues in the FDA and industry are currently validating the CiPA approach at different levels: voltage protocols, instruments and stem cells. ChanTest is meeting the CiPA implementation challenge with the Cardiac Ion Channel Panel, SaVety assessment of Multiple Ion Channel Effects (MICE), and human stem cell–derived cardiomyocytes assays. Validation of the CiPA approach is proceeding at a feverish pace, with the goal of reaching finalization by 2016.
Carlos Obejero-Paz, M.D., Ph.D. ([email protected]), is a principal scientist at ChanTest Discovery Services, Charles River.
1 Kramer J, Obejero-Paz CA, Myatt G, Kuryshev YA, Bruening-Wright A, Verducci JS, Brown AM, MICE models: superior to the HERG model in predicting Torsade de Pointes, Sci Rep., 2013;3:2100 ?
2 Towart R, Linders JT, Hermans AN, Rohrbacher J, van der Linde HJ, Ercken M, Cik M, Roevens P, Teisman A, Gallacher DJ, Blockade of the I(Ks) potassium channel: an overlooked cardiovascular liability in drug safety screening?, J Pharmacol Toxicol Methods., ?
3 Lacerda AE, Kuryshev YA, Chen Y, Renganathan M, Eng H, Danthi SJ, Kramer JW, Yang T, Brown AM., , J Pharmacol Exp Ther., 2008;324:427-33 ?
4 O'Hara T, Virág L, Varró A, Rudy Y., Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation, PLoS Comput Biol., 2011;7:e1002061 ?
5 Mirams GR, Cui Y, Sher A, Fink M, Cooper J, Heath BM, McMahon NC, Gavaghan DJ, Noble D., Simulation of multiple ion channel block provides improved early prediction of compounds' clinical torsadogenic risk, Cardiovasc Res., 2011; 91:53-61 ?
This article is based on a Charles River Eureka blog post that appeared in May.