
In December 2019, a novel virus crossed into humans and has gone on to cause global havoc in the intervening months. At the time of writing in August 2020, the betacoronavirus SARS-CoV-2 (2019-nCoV), causative agent of COVID-19, has spread to every continent except Antarctica, with more than 20.4 million confirmed cases and regrettably over 740,000 deaths and rising. Despite the emergence of zoonotic viruses on a regular basis (for example, SARS in 2003, swine flu in 2009, and MERS in 2012) and numerous warnings of the potentially devastating consequences of a prophesized “once in a hundred years” event, many countries across the world were largely unprepared for such an outbreak. Although the unparalleled global scientific response has been remarkable to witness, we believe it could have been faster had the appropriate systems and regulations been in place.
A pandemic must be fought on three fronts. First, diagnostics are needed. You must be able to identify those patients who have the disease, with an antigen test, and those who have had it, with an antibody test. Second, therapeutic drugs are required to treat those patients that are critically ill. These could be repurposed drugs that had been developed to treat an alternative disease, as we have seen with remdesivir, or novel ones developed rapidly for the new virus. Third, a vaccine must be developed and distributed worldwide as part of a long-term strategy to generate global herd immunity.
On each front, it is clear that there is a requirement for very rapid production of biological reagents, either to go into testing kits or clinical trials. Yet in each of these three areas, the typical manufacturing processes take months to be established.
Antibody manufacturing for diagnostics, therapeutics, and vaccines
The manufacturing of antibodies, or other proteins, can be broken down into five categories, as shown in Figure 1. In the in vitro diagnostics (IVD) market, most antibodies are polyclonals, derived from animal immunization, or monoclonals from hybridomas. Both techniques offer low-cost solutions for antibody manufacturing, but they are difficult to scale rapidly. A goat might yield 200 mg of specific antibody and a hybridoma 10–100 mg/L of cell culture.
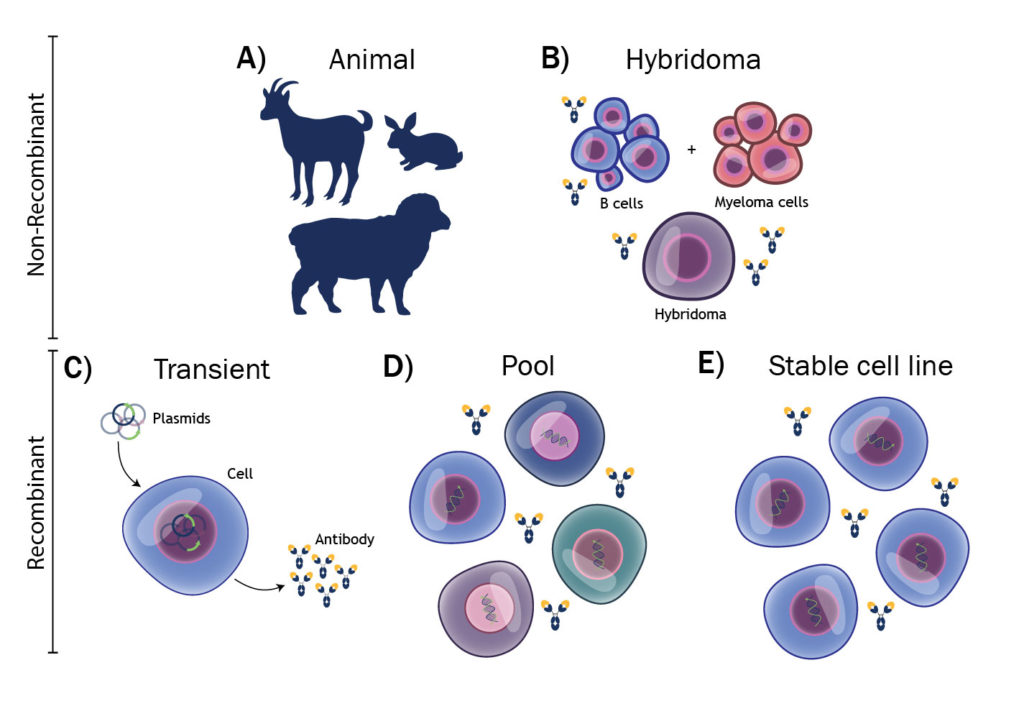
To put this into perspective, our estimates are that approximately 1 kg of antibody would be required to make lateral flow antibody testing devices for 1 billion people. This is without even considering the challenges of reproducibility with nonrecombinant antibodies.1 The prospect is even more daunting when considering the need for SARS-CoV-2 antigens, which are often more difficult to produce than antibodies.
Unlike in IVD, in the therapeutics arena, recombinant antibody production is the norm. But in a pandemic situation, speed as well as scalability is vital. Therapeutic antibodies are traditionally produced from clonal cell lines, typically Chinese hamster ovary (CHO), which take 6–12 months to develop before production can be scaled up in bioreactors. Only now, eight months after a pandemic was declared, are we starting to see therapeutic antibodies being put into the clinic. This in itself is an astonishing achievement, but at a time when speed is so critical, one can’t help but wonder if we could have gone quicker.
Vaccine development has moved at a remarkable pace, with clinical trials starting in early March. However, many of the front-running vaccine candidates are very novel, and it remains to be seen how successful they will be, how quickly they can be manufactured at scale, and when they will become available for mass immunization. As of May 2020, there were well over 100 vaccines in development, with the most popular approach being protein subunit vaccines.2 Despite their popularity, only one protein-based vaccine has made it into clinical trials to date.
One of the many challenges for recombinant protein–based vaccines is the rapid scaling of production, often due to low yields. Using existing antibody production infrastructure offers a potential alternative via the fusion of viral antigens with an Fc domain.
Almost 10 years ago, researchers demonstrated that these Fc fusions can generate immunity in animals even in the absence of adjuvant.3 This approach also has the distinct advantage over many other vaccine approaches of scalable manufacturing using tried and tested CHO stable cell lines. Despite the potential advantages, this concept does not appear to have gathered much traction in recent years, although a Chinese consortium has recently shown CHO-expressed SARS-CoV-2 S1-Fc to be a strong candidate for vaccine development based on nonhuman primate studies.4
Moving toward transient expression
The established manufacturing infrastructure for protein-based reagents, vaccines, and therapeutics is either too slow to set up or insufficiently scalable to be of immediate use in a crisis. That being said, we do have an established technology that can contribute. It is transient expression, or gene expression following transient transfection.
Transfection is the process of introducing foreign genetic material into cells by chemical, biological, or physical processes. In stable transfection, stable clonal cell lines are generated through the introduction of foreign DNA into cells, months of selection pressure, and clonal selection and expansion. Stable transfection may be used by a biologics manufacturer to ensure that a protein-encoding gene of interest is integrated into the genome of cells, which then express the encoded protein at high levels for extended periods.
In transient expression mode, there is no selection for integration, and the protein is expressed only for a short period of time, typically 7–14 days. Methods for transient expression of antibodies have improved markedly over the last two decades with antibody yields increasing from low mg/L levels to as much as 3 g/L.5 Although transient expression is often thought of as a preclinical research activity, it has been scaled to at least 100 L.6 With modern g/L-level transient expression systems, this would represent a useful quantity of material, whether it be for proof-of-concept clinical trials or IVD manufacturing.
Indeed, transient expression beyond preclinical research is exactly what others have proposed in the past.7,8 If the technology exists, why aren’t we utilizing it? The biggest challenge is regulatory. The latest guidance from the U.S. Food and Drug Administration specifically requires a clonal cell line, thus prohibiting transient production.
This guidance was published in 1997, long before transient production of the quantities of antibody required for clinical use was feasible. The guidance is outdated and at odds with the fact that other types of biotherapeutics currently in the clinic, such as virus-like particles and adenoviruses, are manufactured using nonclonal transient expression systems.
Although the technology is well-established in preclinical biopharmaceutical research, it has rarely, if ever, been performed at scale in a GMP facility. Existing therapeutic antibody manufacturing facilities are built around the tried-and-tested cell line development process, but much of the same equipment and facilities could be used in transient mode.
For IVD, the switch to transient expression would be more radical as almost all IVD antibodies are still produced via nonrecombinant technologies. Regrettably, this mindset of not adapting to more modern antibody manufacturing processes has hindered the IVD community in its response to COVID-19, with many antibody manufacturers struggling to keep up with the ever-increasing demands from developers of ELISA and lateral flow antibody tests.
Lastly, there are concerns around safety. To the best of our knowledge, transient material has never been used in the clinic. There would, rightly, be concerns around reproducibility, especially with respect to glycosylation, but with the appropriate controls and analytics, this should be manageable. Indeed, reproducibility is also an issue with classical cell line development processes, and it is well established that a clonal cell line is far from uniform.9
Transient expression in a pandemic
With transient expression, you can go from gene sequence to purified antibody, potentially in significant quantities, in under two months. It may sound farfetched to propose this “research grade” production technology for large-scale manufacturing, but in a five-week period in March and April of this year, scientists at Absolute Antibody produced multigram quantities of multiple anti-SARS-CoV-2 spike protein–neutralizing antibodies. Some of the antibodies were destined for the IVD market as controls in serology tests, and others were developed as potential therapeutics.
The antibodies, of course, were not to GMP grade, but the production technology that produced them clearly demonstrates that, with the correct funding and infrastructure, rapid transient production of 10–50-g batches of antibody is well within the realm of possibility. Such quantities should be sufficient for Phase I studies to be started rapidly while the more traditional cell line development process continues in the background to enable the production of the larger volumes needed for later-stage studies.
In theory, transient expression technology could allow a clinical trial to start within two to three months of an antibody sequence being identified. Although this application of the technology would be a radical change from current industry standards, it is exactly what is required in a global crisis.
References
1. Bradbury A, Plückthun A. Reproducibility: Standardize antibodies used in research. Nature 2015; 518: 27–29.
2. Callaway E. The race for coronavirus vaccines: a graphical guide. Nature 2020; 580: 576–577.
3.Loureiro S, Ren J, Phapugrangkul P, et al. Adjuvant-Free Immunization with Hemagglutinin-Fc Fusion Proteins as an Approach to Influenza Vaccines. 2011; J. Virol. 85: 3010–3014.
4. Ren W, Sun H, Gao GF, et al. Recombinant SARS-CoV-2 spike S1-Fc fusion protein induced high levels of neutralizing responses in nonhuman primates. Vaccine 2020; 38: 5653–5658.
5. Daramola O, Stevenson J, Dean G, et al. A high-yielding CHO transient system: coexpression of genes encoding EBNA-1 and GS enhances transient protein expression. Biotechnol. Prog. 2014; 30: 132–141.
6. Girard P, Derouazi, Baumgartner G, et al. 100-liter transient transfection. Cytotechnology 2002; 38: 15–21.
7. Stuible M, van Lier F, Croughan MS, Durocher Y. Beyond preclinical research: production of CHO-derived biotherapeutics for toxicology and early-phase trials by transient gene expression or stable pools. Curr. Opin. Chem. Eng. 2018; 22: 145–151.
8. Kelley B. Developing therapeutic monoclonal antibodies at pandemic pace. Nature Biotechnol. 2020; 38: 540–545.
9. Tharmalingam T, Barkhordarian H, Tejeda N, et al. Characterization of phenotypic and genotypic diversity in subclones derived from a clonal cell line. Biotechnol. Prog. 2018; 34: 613–623.
Ian Wilkinson, PhD, is Chief Scientific Officer at Absolute Antibody.


