May 1, 2012 (Vol. 32, No. 9)
MolMed Exploits a Tumor-Homing Peptide and a Herpes Gene to Fight Cancer
MolMed (short for Molecular Medicine) has two anticancer therapies in Phase III clinical trials. The candidates are based on distinctly different technology platforms.
MolMed, headquartered in Milan, Italy, at the San Raffaele Biomedical Science Park, is developing a first-in-class vascular targeting agent that selectively homes in on the tumor vasculature. The agent, called NGR-hTNF, uses a tumor-homing peptide (NGR) to deliver human cytokine tumor necrosis factor (hTNF) to slow or block tumor progression.
The technology originated through a collaboration with the Burnham Institute, where peptides that bind only to human vascular tissue were discovered in phage display libraries. The highly specific NGR was selected, and researchers at the San Raffaele Institute fused it to hTNF.
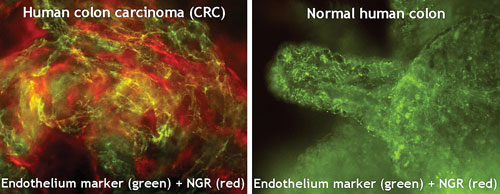
NGR-hTNF targets the blood vessels that nourish tumors through specific binding of both moieties of the molecule to the neovasculature endothelium. Animal models confirm that NGR-hTNF directly acts on the vasculature to suppress tumor growth. NGR-hTNF shows broad therapeutic potential for the treatment of very different types of solid tumors, both common and rare, according to the company.
Clinical trials started in 2003, and since then, clinical investigators have obtained evidence of antitumor activity in a series of tumors including colorectal cancer, liver cancer, ovarian cancer, small-cell lung cancer, soft tissue sarcomas, and mesothelioma.
NGR-hTNF is now being investigated in pivotal Phase III trials for malignant pleural mesothelioma. An IND was recently cleared by the FDA to include clinical centers in the U.S. NGR-hTNF also is in Phase II trials for six more solid tumor types.
It’s been known since the 1980s that TNF has potent antitumor activity. However, patients treated with TNF alone experience severe toxicity side effects. “We took a different approach by attaching TNF to a peptide that delivers it directly to tumors for maximal antitumor activity,” says Claudio Bordignon, M.D., a hematologist and the firm’s CEO.
This provides powerful antitumor activity at doses that are 100 times lower than the known toxic dose of TNF, Dr. Bordignon reports. Because of the tiny doses needed, TNF shows no significant toxicity issues in patients. In fact, no toxicity has been reported for patients with non-small-cell lung cancer treated for up to two years with NGR-hTNF, he notes.
NGR-hTNF also works well in combination with chemotherapy agents, such as cisplatin or doxorubicin. “There’s a specific rationale for giving NGR-TNF with chemotherapy,” explains Dr. Bordignon. In the first few hours after injection with TNF, capillary leakage increases, which, in turn, promotes penetration of chemotherapy drugs into the tumor.
The protocol for using NGR-hTNF in non-small-cell lung cancer is to treat patients with first-line chemotherapy in combination until the maximum tolerated dose of the chemotherapy agent is reached. Then NGR-hTNF is continued alone as a maintenance therapy. When all other treatments options are exhausted for a patient, NGR-hTNF can be added as a maintenance therapy.

Specific, NGR-mediated binding of NGR-hTNF to tumor blood vessels: NGR binds to tumor vessels of human CRC and not to those of normal intestine. (NGR-Quantum dot administered in vivo: tissue samples from same patient.)
Improving Bone Marrow Transplants
MolMed scientists also work in the field of genetic modification of hematopoietic stem cells. The company says its cellular therapy product, based on genetically engineered donor lymphocytes, allows leukemia patients to receive bone marrow transplants from partially compatible donors, and it eliminates the need for immune-suppressing drugs. The method works especially well for bloodborne tumors like acute leukemia, Dr. Bordignon remarks.
He explains that researchers at MolMed invented the technology, known as the TK platform. The method consists of inserting the thymidine kinase (TK) gene of herpes simplex virus into the lymphocytes of donors, which are infused into the patient after bone marrow transplantation. The antiviral agent downregulates cells and prevents toxicity and immune suppression. “Patients with acute leukemia who do not have compatible donors now can receive transplants,” says Dr. Bordignon.
Currently, a bone marrow donor must be fully compatible with a patient, a condition that is satisfied only for about half of transplant candidates. TK is the only therapy that specifically addresses the issue of delivering safe and effective hematopoietic stem cell transplantation from a half-compatible donor to treat high-risk leukemia patients, claims Dr. Bordignon.
If standard transplants are performed using donors who are only half-identical, severe graft-versus-host reactions occur. To prevent this, patients must be dramatically immunosuppressed with drugs, which increases the risk for infections and death. “Thanks to our technology, no immune suppression drugs are needed, only the TK gene,” he says.
European medical centers are evaluating the TK technology in a pivotal Phase III trial. MolMed received IND approval from the FDA for the TK therapy, and the company plans to start enrolling patients in 2011 for U.S. trials at the Northwestern University Medical School in Chicago, and elsewhere.
MolMed’s service sector, GMP Solutions, offers GMP manufacturing services for cellular and gene therapy. The firm is helping Fondazione Telethon (similar to the Jerry Lewis MDA Telethon) develop and manufacture gene therapy treatments for six rare genetic diseases that have no adequate cures.
A single defective gene causes all six of the diseases—metachromatic leukodystrophy, Wiskott-Aldrich syndrome, beta-thalassemia, mucopolysaccharidosis type 1, globoid leukodystrophy, and chronic granulomatous disorder. Therefore, inserting the correct form of the gene into the patient’s own stem cells derived from bone marrow could potentially cure the illnesses.
MolMed develops and produces clinical-grade viral vectors carrying relevant therapeutic genes and manufactures patient cells for investigation in clinical trials. “We collaborate with institutions and charities to make reagents for gene and cell therapy and stem cells for clinical trials,” says Dr. Bordignon.
Larger companies have noticed both of MolMed’s technology platforms, he adds. To advance the NRG-hTNF technology to treat solid tumors “we will need partners with strong marketing and distribution capabilities,” he says. The TK technology is more limited and MolMed may develop that therapy on its own.