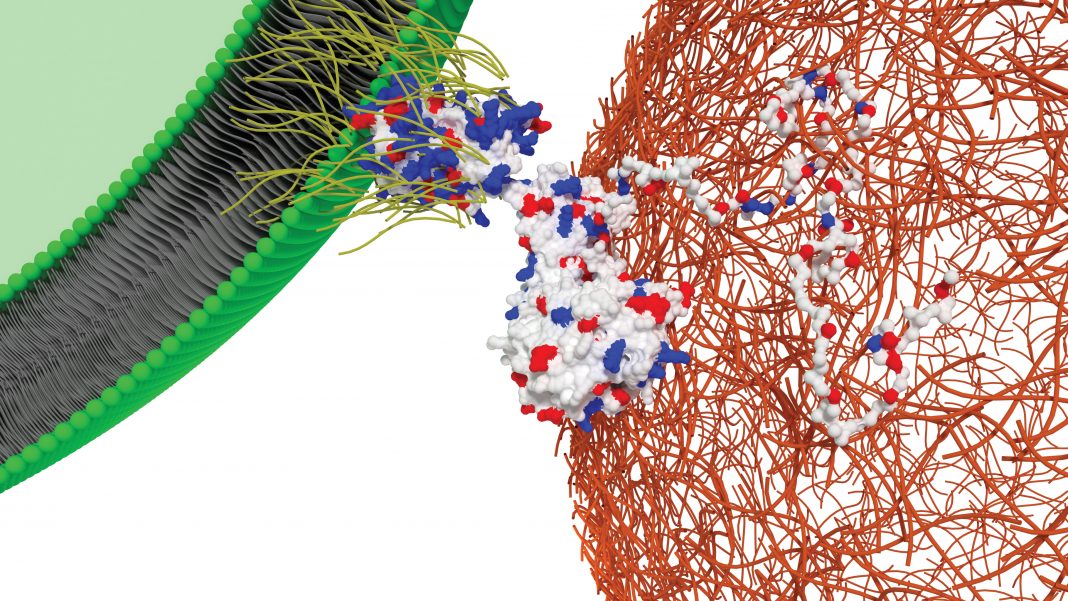
Synthetic biology is among the most exciting emerging technologies. By taking an engineering approach to living systems, synbio—as synthetic biology is often called—aims to design and build new biological products and organisms to overcome some of humanity’s biggest challenges.
Synbio enthusiasts claim that the technology could transform industries as diverse as petrochemicals and pharmaceuticals—helping people remain healthy, improving food production, and even generating renewable energy. Some of these enthusiasts enjoy considerable influence. In the United Kingdom, for example, they contribute to the work of the Technology Strategy Board (TSB) and the Department of Business, Industry, and Skills (BIS). In 2011, BIS issued a report indicating that TSB had identified synbio as “a key emerging technology with the potential to create a billion-pound industry within the United Kingdom in the next decade.”
Last June, SynbiCITE, the United Kingdom’s national center for the commercialization of synthetic biology, held a conference at the Queen Elizabeth II Centre in London. SynbiTECH 2019 aimed to highlight the “key opportunities and challenges for building a multibillion-dollar synthetic biology industry.” Speaking in a session on synbio in healthcare, UK startups presented new microbe-based drugs as well as artificial proteins for delivering medications to cells.
Getting to the guts
Synbio research would have us reconsider what we mean by “drug.” Traditionally, a drug has been a therapeutic molecule. But a drug could be a drug system, a discrete package that combines a drug with mechanisms for its production and delivery.
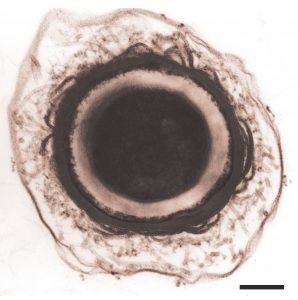
An expansive view of design has been adopted at CHAIN Biotechnology, which is developing new therapeutics based on living microbes where the “bug is the drug.” These drugs are based on a single strain of Clostridium bacteria that is naturally found in the gut, but which has been deliberately engineered to carry a therapeutic payload, such as custom metabolites, enzymes, or peptides. The therapies are taken as capsules for oral ingestion with the aim of treating chronic and debilitating diseases associated with the gut including inflammatory bowel disease and C. difficile.
“There’s a massive opportunity in the gut microbiome,” says Edward Green, PhD, founder and chief executive of CHAIN Biotech. “Microbial-based therapeutics are evolving, and synbio is playing a major part. What it gives you are better understood mechanisms of action leading to more efficacious drugs.
“Microbiome therapeutics offer significant patient benefits. Not only do they address unmet medical needs, they do so in a very cost-effective way with improved safety. Oral delivery means it’s simple to administer and puts the patient in control rather than rely on clinicians and hospital visits.”
According to Green, CHAIN Biotech is currently completing preclinical work on a product for ulcerative colitis, but it is also developing other products by engineering its bacterial “chassis” (the synbio term to describe the microbe delivering the drug). The next stage will be to take one or more products into clinical trials to demonstrate safety and efficacy in humans.
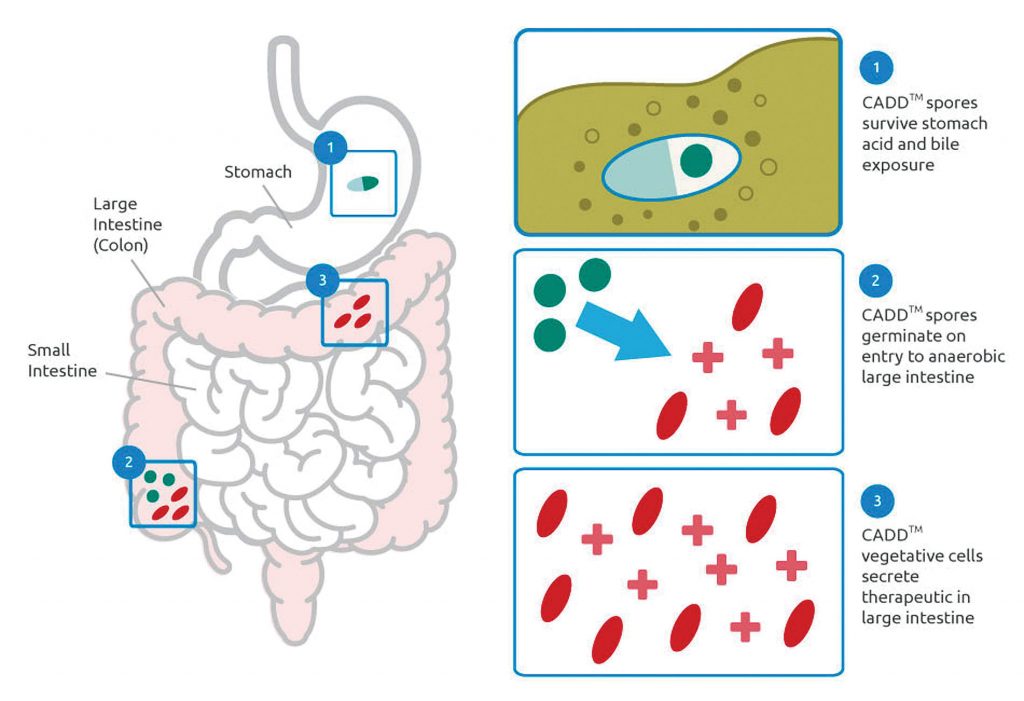
Although it is early days, he expects interest in microbiome therapeutics to build. “The use of engineering,” he explains, “provides an exciting growth opportunity for synbio.”
Several companies are engineering different chassis for microbiome applications, but—according to Green—CHAIN Biotech is the only one to focus on the Clostridia class of bacteria. The main advantage, he claims, is that bacteria of this class have many growth characteristics that make them particularly suitable for targeted drug delivery. He adds that new gene editing tools like CRISPR “open up exciting engineering options for this important pclass of microbe.”
Moving toward the market
The engineering of bacteria also occupies Prokarium, a company that has several products in its development pipeline. “We began working on vaccines in 2012,” says Ted Fjällman, PhD, the company’s chief executive officer. Initially, the company bought intellectual property from companies based in the United States and combined it with intellectual property from companies based in the United Kingdom. Prokarium’s aim, Fjällman points out, was to “prove we could use bacteria as a vaccine delivery system.”
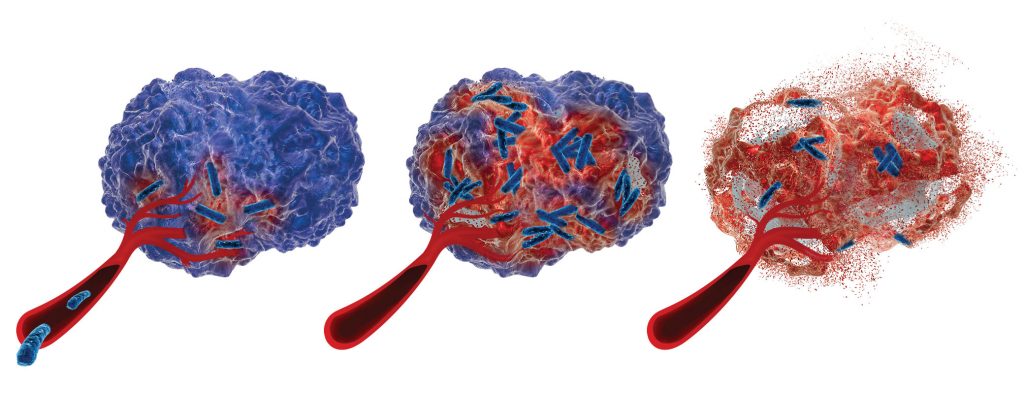
He explains some of the benefits. A vaccine delivered directly to the gut doesn’t circulate in the bloodstream. Avoiding the circulation reduces side effects, and—if the drug is also acting as the bioreactor—it obviates the need for complex purification. The chassis organism used by Prokarium has a good safety record, Fjällman maintain. In fact, this organism has already demonstrated safety in 10 clinical trials in the United States, the United Kingdom, and Vietnam.
However, there are also disadvantages to developing engineered bugs as drugs. “Our work,” explains Fjällman, “is very cutting edge. There’s only one genetically modified organism approved as vaccine in the world.” (This vaccine, Vaxchora™, is available from Emergent BioSolutions. It has been approved by the FDA for the prevention of cholera.) Part of the challenge with bugs as drugs is regulatory. Companies need to prove the mechanism of action and the safety of live genetically modified organisms—which not only need to be safe for patients but incapable of spreading if released into the wider environment.
Although live bacterial vaccines have been used before, they were created by mutagenesis, which isn’t regarded as genetic engineering. According to Fjällman, however, Prokarium is ahead of the game, as the bacteria used by the company are specific to humans and can’t survive in pets or outside a human host. The company expects that its lead vaccine candidate, Entervax™, will enter clinical trials in 2020.
Around 18 months ago, Prokarium raised $10 million and brought on two new investors interested in using the company’s platform to develop immunotherapy for solid tumors. “The early data [are] exciting,” proclaims Fjällman. “The idea is that the bacteria colonize the tumor and grow there, attracting the immune system to attack tumors that otherwise wouldn’t be recognized.”
Improving cell therapies
“We’re attacking a set of problems that all cell therapies have,” says Adam Perriman, PhD, professor of bioengineering at the University of Bristol and founding director of CytoSeek. “[We’re addressing] immunomodulation in T cells in CAR T-cell therapy. We look at oxygenation of the transplanted cells, and we look at homing and retention—once injected, we can get them to the damage site.”
The company was incorporated in 2017 and aims to improve cell therapies, such as CAR T-cell therapies, where a patient’s own T cells are engineered to fight cancer. The company has used the engineering design techniques of synthetic biology to create an artificial membrane-binding protein, which self assembles at the surface of a T cell or other mammalian cell. The constructs constitute a protein surrounded by a surfactant mesh, fused to another protein or enzyme designed to have a specific benefit for the CAR T cells.
“You can plug and play any protein or enzyme, within reason,” he explains. “That’s why we’re [operating as] a technology company rather than supplying any single therapy.” The idea is to incubate the T cells with the artificial protein at the end of the CAR T-cell process, shortly before the engineered cells are frozen for transport back to the patient. The aim is that the protein attached to the construct can add oxygen to the T cells in the hypoxic environment of a solid tumor or have a positive effect on the immune response.
The technology is also applicable to heart patients or those with skeletal damage, where the company can add a homing molecule to a membrane-binding protein. The idea is to deliver oxygen or other molecules directly to the damaged area. “We started off with a small Innovate UK Smart grant that was supported by venture capital, funds from SynbiCITE, and university-based funding,” Perriman explains, “but we’re starting to get more serious now.”
The company, which is closing a seed round of funding worth in excess of a million pounds, is working on in vitro–stage research and is planing in vivo experiments with mouse models. As CytoSeek is a technology company, it’s discussing an early partnership with a cell therapy specialist. “For us,” Perriman notes, “it’s about augmenting and solving problems in cell therapy.”
Creating synthetic DNA
Touchlight Genetics is working on improving gene therapy, according to R. Michael Linden, PhD, head of the company’s scientific advisory board. The company is jointly building a manufacturing facility in Spain with AskBio, a company using adeno-associated viruses (AAVs) to deliver gene therapy.
Touchlight has developed a patented method for producing synthetic DNA without using bacteria, which—according to Linden—offers a huge potential benefit to AAV manufacturers. “There can be [a] six-month wait for viral DNA,” he explains. “And now that gene therapy is a commercial activity, time and quality are of the essence.”
Synthetic DNA has none of the problems of DNA created using bacteria, such as bacterial genes for antibiotic resistance being incorporated into the AAVs by accident. “If the manufacture of AAV using synthetic DNA takes off, it could be quite disruptive to the entire industry,” Linden asserts. “Regulators will think, ‘gee whiz’—and force everyone to switch to Touchlight’s technology.”

As DNA is the active substance in gene therapy, the company is also at the early stages of working out how to turn synthetic DNA into a drug. “We’re aware,” says Linden, that “delivery is a question. How do you deliver DNA in vivo to a patient [without using a virus]?” If Touchlight finds the answer, the company’s technology could have many applications, including prophylactic immunization and the delivery of gene therapy to a patient’s organs.
In addition, synthetic DNA can be repurposed as a template for manufacturing mRNAs for vaccines and other therapies. According to Linden, “In traditional manufacturing, there’s a lot of rearranging of template DNA to make these mRNAs so you have a heterogeneous, rather than homogeneous, drug product.” With synthetic DNA, he explains, it’s possible to manufacture long stretches of DNA that repeat a single base and don’t degrade over time.
Boosting wound care
“What’s exciting about synbio is the range of possibilities it opens up,” says Martin Challand, PhD, chief technology officer and co-founder of Zentraxa. The company, which did not present at SynbiTECH 2019, is drawing inspiration from the natural world to create biopolymers that don’t exist in nature. The company aims to develop new adhesives that, due to their biological basis, have a strong adhesion to skin for better wound or ostomy care. “We’re working on bonding in wet environments,” Challand explains, “and the ability to programmably debond.”
Originally inspired by work at the University of Bristol, the company spun out in March 2017 and is currently doing feasibility studies on the technology. Although excited by the possibilities of synbio, Challand warns that the sector faces a challenge in translating research and scaling up from the laboratory.
“People have a good handle on how to exploit biology to create product prototypes,” he points out. “But to make it commercially viable, it needs to be made at an appropriate cost and scale.” He believes that Zentraxa’s unique selling point is the methodology the company uses to exploit its synbio product. Zentraxa, he says, is looking at cost of manufacture and sustainability at a very early stage.