January 15, 2011 (Vol. 31, No. 2)
Hopes Are High, Yet Potential Pitfalls Abound as Research Wends Its Way through the Clinic
The future may not exactly rest in the figurative hands of stem cells, but David Magnus, Ph.D., director at Stanford Center of Biomedical Ethics and professor of medicine and biomedical ethics, pediatrics at Stanford University, acknowledges that the stakes are high. “There are tremendous expectations of stem cell research and what it can mean for personalized medicine,” he says.
“The public and many patients want treatment, and they want it today. The downside of the hype is that clinical trials take time, and people want answers now,” Dr. Magnus adds.
“If only we had a working crystal ball and could see what’s coming—which trials are going to succeed. But this is the challenge,” says Robert J. Deans, svp, regenerative medicine, Athersys. “This is a high-profile area of research. When things succeed, they raise hopes among researchers, the public, and, of course, among investors. When they fall short, they also dramatically impact expectations across the board. The benefits can be incredible, but so are the risks, particularly when you get to the pivotal clinical trials stage.”
These themes will loom large at CHI’s upcoming CHI “Molecular Medicine” conference. The meeting will focus heavily on the practical and regulatory ramifications of moving stem cell research through the clinic and drill down into some of the research currently moving through the clinic.
Basic research in stem cells is moving forward quickly, Dr. Magnus notes. “There is a lot happening in the field with regard to differentiated cells—for example, the phrase ‘stem cells’ is often applied to bone marrow transplantation or transplantation of primary cells derived from bone marrow,” he says.
“I will be concentrating on the use of far less-established applications including use of human embryonic stem cell (hESC)-derived stem cells. The real challenges in this field are at the frontier—not standard, well-established areas of research.”
One big challenge to the future of stem cell research, says Dr. Magnus, is an obvious one—specifically, to find the funding to do the research. “State funding and CIRM money is going to be ending in a few years. The amount of federal funding is not growing, and there are legal and political challenges to what can be funded. Largely, states will be responsible for funding, and having that funding slow down also presents a challenge,” he reports.
“The legal challenge is how will NIH fill the funding gaps, as there is a huge monetary limitation in the field. Also, you look at the political landscape and wonder how a promising young scientist is going to find his or her way, as there are limits to the cell lines you can use. Researchers can’t always get funding because of the political landscape. And as we move forward with clinical trials, we are nervous about how high-profile clinical trials are going to go.”
Of major concern, says Dr. Magnus, are the incredibly high public expectations. “Public expectation is unrealistic, and when you throw in a high-profile death, we don’t want what happened to gene therapy to happen to stem cells,” he cautions. “Jesse Gelsinger’s death caused the gene therapy field to take a major hit, from which it still hasn’t recovered. Stem cell research would do well to avoid such a tragedy. At the frontiers, the risks and the rewards are the biggest.”

GFP-expressing multipotent adult progenitor cells (green) modulate the negative effects of macrophages (purple) after experimental spinal cord injury. [Sarah Busch/Athersys]
Spinal Cord Injury
“This field has moved quickly in development,” notes Dr. Deans. “Over the last several years, the field has moved into pivotal clinical trials. At Athersys, we’ve attracted the interest of large pharma on the strength of our proof-of-mechanism studies and a viable path for clinical product development.”
Athersys has been developing Multi Stem®, a stem cell product to treat multiple disease indications including myocardial infarction, hematopoietic stem cell transplant support—for example, in leukemia or lymphoma patients that receive a bone marrow transplant and are at risk for developing graft versus host disease—ischemic stroke, and a range of conditions involving immune system function such as inflammatory bowel disease.
“One of the hurdles overcome in the last few years is scalability,” says Dr. Deans. “Now, stem cells can be manufactured on a large scale; over 100,000 doses can be made from a single donor. This is based on the properties of the cell types we have worked with, and taking a rigorous matrix-based process-development approach. One advantage is that we have contracted out the manufacturing to a facility with the infrastructure that can help us leverage our knowledge.”
Dr. Deans describes an experimental spinal cord injury model wherein the injection of stem cells near the site of injury reverses the effect of inflammatory cells. “We have a model for this, where axons are unable to pass over a proteoglycan matrix,” he says.
“We show that when macrophages are added, axons retreat, but when our cells our added, they cause the macrophages to detach from the axon, and neurons actually grow forward. We’ve proven that this response is mediated by release of metalloproteinase activity from the macrophage, and our cells directly inhibit this. An example in cell therapy where a molecular pathway is attributed to disease pathology was demonstrated in a surrogate in vitro model, and then shown to be relevant in an in vivo injury model.”
The current major hurdle in this field, Dr. Deans says, is understanding the pharmacokinetic/pharmacodynamic (PK/PD) profile of stem cells. Drug makers have a standard of how a drug acts in the body, and we need to know: where do these cells go? How long do they last? What pathways do they use? How long are they effective?” Dr. Deans says.
“Right now, those are the questions that form the last hurdle for investment in this space by big pharma. There is a value and urgency to creating a stronger PK/PD profile. I will endorse this approach to help move candidates to a late-stage clinical trial.”
Immune Targeting of Glioblastoma
Glioblastoma multiforme presents a myriad of challenges, notes John S. Yu, M.D., professor and vice-chair, department of neurosurgery, Cedars-Sinai Medical Center, and chairman and CSO at Immunocellular Therapeutics. “Despite years of advances with glioblastoma, survival remains less than 15 months. One problem is that of cancer stem cells that infiltrate the brain as microsatellites of tumors.”
Dr. Yu was fascinated by the possibility of affecting disease at its root, at the cancer stem cell level. “We have historically targeted the less important daughter cell with radiation and chemotherapy. Cancer stem cells are resistant to these forms of therapy, so targeting cancer stem cells may get at the root of these tumors.”
Dr. Yu’s group utilized a dendritic cell vaccine strategy to target tumor-specific antigens that are overexpressed on glioblastoma cancer stem cells. In a trial with ICT-107, Dr. Yu’s group is targeting six specific proteins in this cell population. Early results, Dr. Yu notes, are promising. “Of 17 glioblastoma patients treated in this Phase I trial, 41 percent of patients developed an antigen-specific interferon gamma response after vaccination,” he says. “The median progression-free survival was over 17 months, and the median survival was not reached to date. The planned Phase II trial will enroll 102 patients.”
hESC-Derived Cardiomyocytes
Companies expanding into the frontier include Geron, which recently launched a clinical trial using hESC-derived oligodendrocyte progenitor cells called GRNOPC1, which is a population of living cells containing precursors to oligodendrocytes. Oligodendrocytes are lost in spinal cord injury, resulting in myelin and neuronal loss that causes paralysis in many patients.
Katharine Spink, Ph.D., vp operations and regenerative medicine programs, will be presenting preclinical results from Geron’s study on GRNCM1, an hESC-derived cardiomyocyte for the treatment of heart failure. This includes methods for high efficiency differentiation of cardiomyocytes from hESCs, characterization of the resulting cell population, preclinical data on cellular function, and steps for the development of this product toward the clinic.
Researchers have demonstrated proof-of-concept in mice, Dr. Spink explains. Mouse embryonic stem cells have been used to derive mouse cardiomyocytes. When injected into the hearts of recipient adult mice, the cardiomyocytes repopulated the heart tissue and stably integrated into the muscle tissue of the adult mouse heart, she says. She believes that in human medicine, it is possible that hESC-derived cardiomyocytes could be developed for cellular transplantation therapy in humans suffering from congestive heart failure and the damage caused by heart attacks.
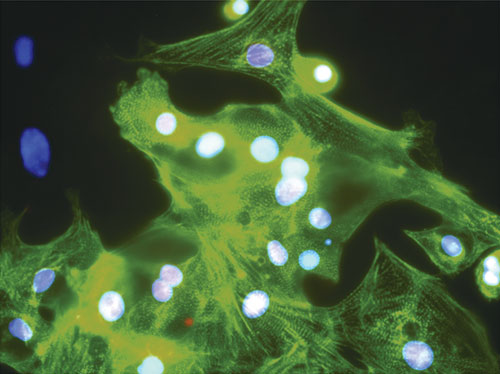
Geron’s GRNCM1 shows expression of cardiac markers alpha-actin and Nkx-2.5.
Myocardial and Critical Limb Ischemia
Autologous stem cell therapy can be defined as an attempt to regenerate and replenish tissue by increasing the supply of naturally occurring reparative cells at sites of damage. This approach relies on the phenomenon of plasticity—autologous stem cells from one tissue generating specialized cells of another tissue.
Douglas Losordo, M.D., director, Feinberg Cardiovascular Research Institute, and professor, heart research, Feinberg School of Medicine, Northwestern University, will be presenting his group’s work in patients with advanced cardiac and vascular disease. “We have results from two randomized, controlled clinical trials of CD34 stem cell therapy for cardiovascular disease,” says Dr. Losordo.
“One shows significant improvements in chest pain and exercise capacity in treated versus controls. The other is a Phase IIa study in patients with critical limb ischemia that shows a significant reduction in amputation rates in patients treated with their own CD34 cells.”
The goal is to spur the growth of small blood vessels that make up the microcirculation of the heart muscle, he says. Researchers believe that the loss of these blood vessels contributes to the pain of chronic, severe angina. “Autologous cells have been used for a long time in transplants, providing some history of safety, albeit in different indications,” says Dr. Losordo. “The challenges are similar, I think, to the development of any novel therapy: establishing proof of principal, evidence for safety, and so on.”
The results seem promising; Dr. Losordo adds, “Phase III studies for cell therapies for cardiovascular disease will be performed in the next 12–18 months.”


