April 1, 2018 (Vol. 38, No. 7)
In Bioprocessing, Changing One Parameter Can Change Every Other Parameter—Often Unpredictably.
Biopharmaceutical companies that develop protein and nucleic acid therapies face unique manufacturing challenges. For example, these companies must create processes, scaleup operations, maintain the optimal balance of in-house and outsourced manufacturing capabilities, and predict future demand.
Manufacturing challenges were on the agenda at the BioProcess International West (BPI West) meeting in San Francisco, where scientists met to discuss possibilities for improving the speed, lowering the cost, and increasing the quality of biologics development.
One of the speakers at the event was Christope Martin, upstream production (USP) manager in the life sciences division of Germany-based Merck & Co. (the life sciences business operates as MilliporeSigma in the United States and Canada). He shared insights gleaned from his experiences with large-scale single-use systems. Single-use production equipment, he noted, has been used at Merck Biodevelopment in France since 2011. In 2015, manufacturing capacity was increased after the creation of new suites dedicated to production with single-use equipment at the 2-kL scale.
Martin said that USP scaleup could incorporate a “gazing strategy.” To illustrate, he described his work with single-use bioreactors for mammalian cell culture. In this work, the strategy involved creating oxygen mass transfer models for maintaining constant oxygenation from 2-L scale (in development) to 2,000-L scale (in production) while accommodating the geometry of whatever system was being used (bag or tank design, impeller, etc.).
Rationalizing Oxygen Supply
Martin explained that the new USP scaleup strategy in particular allows biomanufacturers to rationalize oxygen supply in a bioreactor with real cell oxygen needs. The main elements of the modified process include lower oxygen supply and improved oxygen regulation profile of the culture. Noting that this approach was applicable to all cell types, he said that it was specifically designed for production of therapeutic molecules (such as monoclonals) from CHO cell lines at high density.
From a process point of view, he noted that the strategy is particularly useful for single-use manufacturing. “The strategy allows manufacturers to surpass the potential process limits inherent to single-use bioreactor design,” he explained. “Moreover, it is well aligned with the intrinsic constraints of single-use bags such as the acceptable working pressure.” Relevant influences, he added, include reduced gas supply, lower foam, and fewer issues with gas release.
From a business point of view, Martin noted that the strategy is particularly suitable to single-use manufacturing because it allows companies to reduce the cost of gas installations around a single-use suite.
Design of Experiments
A popular chromatography technique is mixed-mode chromatography (MMC). In MMC, solutes interact with a stationary phase through more than one interaction mode or mechanism. As the biopharmaceutical industry has continued to adopt MMC, the chromatography industry has developed more MMC resins that go beyond traditional supports, enabling protein adsorption by a combination of ionic interactions, hydrogen bonds, and/or hydrophobic interactions, exploiting a combination of both ionic and hydrophobic characteristics of antibodies and contaminating proteins.
Currently, commercially available mixed-mode resins include CHT ceramic hydroxyapatite, CFT ceramic fluoroapatite, and Nuvia cPrime (from Bio-Rad Laboratories); Capto MMC, Capto adhere, and Capto Core 700 (from GE Healthcare); and HEA Hypercel and MEP Hypercel (from Pall).
During the BPI West meeting, Xuemei He, Ph.D., manager of chromatography media chemistry at Bio-Rad, addressed best practices and guidelines for design of experiments (DoE) using an intuitive approach to method development and process optimization. Dr. He included an overview of mixed-mode chemistries, an introduction to DoE, and an analysis of purification results and scaleup using the Bio-Rad NGC Next Generation Chromatography system. Dr. He also detailed a hands-on DoE with Nuvia cPrime mixed-mode media for the purification of a recombinant protein produced in an Escherichia coli expression system.
Asked what specific challenges MMC tackled, Dr. He emphasized that mixed-mode purification is isorthogonal to other separation methods. In general, except for novel mixed-mode supports such as hydroxyapatite, it is not a matter of simply “combining” two different interaction types. “It is,” Dr. He explained, “a matter of combining them in a particular spatial orientation dictated by the ligand that provides the unique selectivity for target protein molecules.”
Because the interactions can be complex, and because the chromatographic behavior of a target protein molecule cannot be easily predicted by its isoelectric point, some form of DoE is useful, she noted. As one example, ion exchange/hydrophobic mixed-mode supports generally use increasing salt to elute from the ionic interaction, but increasing ionic strength generally increases a protein’s interaction with the hydrophobic part of the ligand, changing its conformation and/or aggregation state.
The most frequent challenge to adapting MMC is a lack of understanding of how mixed-mode interactions work. “There’s nothing new here—if you understand the principles of ion exchange, and you understand the principles of HIC [hydrophobic interaction chromatography], then you have everything you need to know,” Dr. He insited. “It is merely a matter of combining that knowledge into designing a step that allows for appropriate adsorption and desorption from the matrix.”
One key advantage to MMC is the availability of possible alternatives to traditional chromatography media. Users can select from a broad range of separation mechanisms, including mechanisms that make it possible to avoid having to add or remove salt, Dr. He pointed out.
When asked how widely MMC has been adopted by biomolecule purification scientists, Dr. He replied that a PubMed search for MMC yielded over 1,300 results. “By far, the largest adopters of mixed-mode resins have been biopharma and biotech companies, followed by other industries and academic labs which focus on production and purification of biomolecules,” she continued. “The largest market by application of mixed-mode resins is for monoclonal antibodies, followed by recombinant proteins, viruses, enzymes, and biosimilars.”
When discussing MMC scaleup in the DoE context, Dr. He referred to the NGC system. The system’s ChromLab software, she said, “helped many groups to automate their routine purification workflows as well as to develop multidimensional chromatography methods. [It’s] flexible, modular and economical design makes it a good choice for method development and scaleup.” The software, Dr. He asserted, provides an intuitive interface and simplifies the exploration of chromatography variables.
“In general, what can vary in chromatography are the buffers you use, their salt concentrations, the isoelectric point, and the amount of protein loaded onto a column,” stated Dr. He. “You can explore these variables to find the optimal operating range. By feeding the operating ranges into the software, you can determine the specific experiments you need to do to find out how the protein will behave on that resin at a given pH or salt concentration. This will help in determining the optimal purification conditions for that particular protein on that resin.”
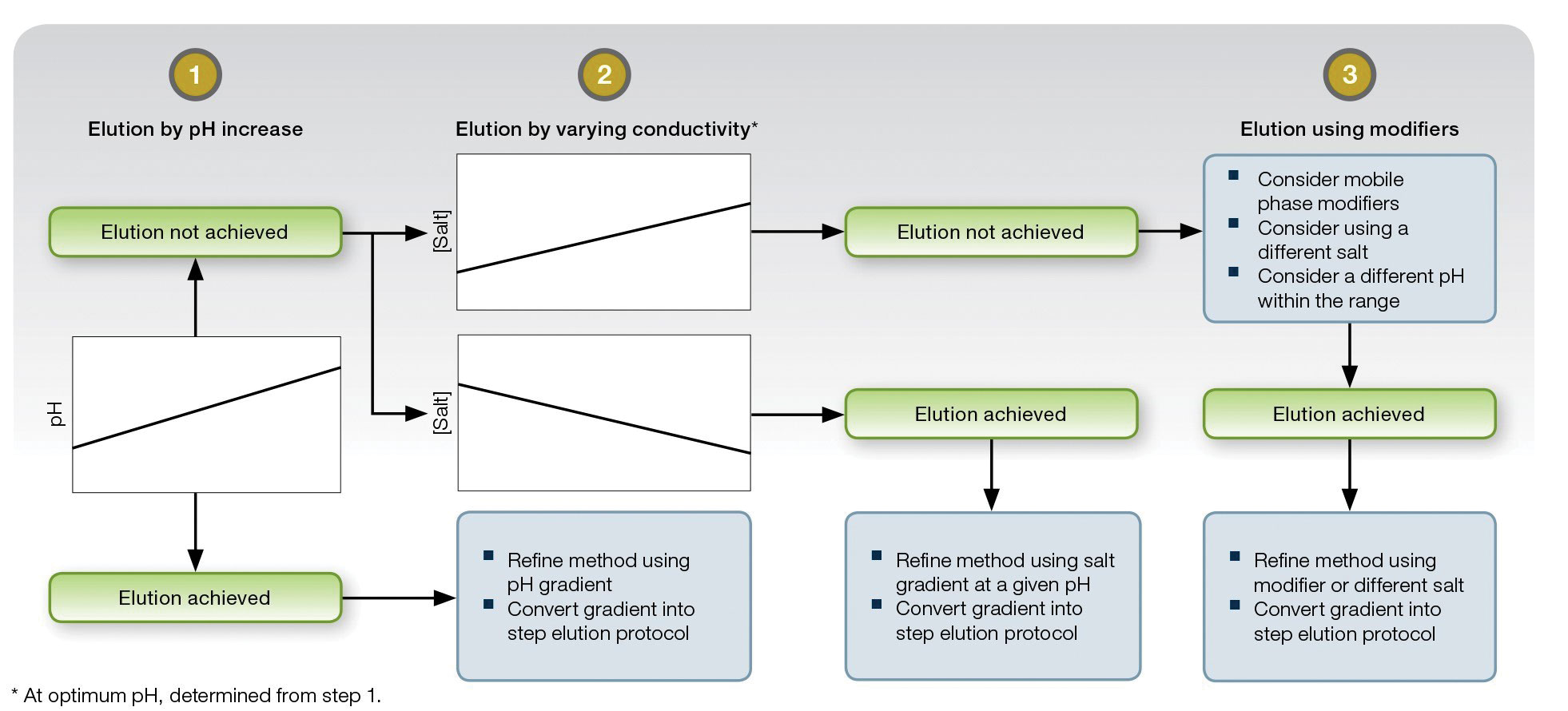
This schematic outlines an approach to method development with Bio-Rad Laboratories’ Nuvia™ cPrime™ media. In most cases, the company explains, a few experimental designs can help a biomanufacturer identify binding and elution conditions that will yield a robust and scalable method. Notice that the binding and elution mechanisms are determined chiefly by pH and salt.
Implementing Process Intensification
Process intensification includes goals such as reducing the process time of a unit operation, decreasing the overall process time by improved alignment of various unit operations, and reducing plant idle time by improved equipment utilization. These goals were the focus of a panel discussion entitled, “Implementation of Process Intensification in Commercial Manufacturing: Drivers and Challenges.” It was sponsored Sartorius Stedim Biotech, which contributed some of the panel’s participants, including Fritjof Linz, Ph.D., the company’s vice president of marketing, purification technologies.
GEN asked Dr. Linz how he would define process intensification, a term he prefers to use rather than continuous manufacturing. “It’s very simple. It boils down to the production of more product over a given time,” he said. “For companies wanting to go down that route, this addresses the questions of how can I reduce costs, how can I get more product, and how can I increase flexibility out of a given facility?”
One big change in the biopharmaceutical industry, he noted, has been the amount of product that can now be produced in a smaller facility: “You can realize the same output in a 2,000-L facility that in the past would have required a 10,000-L plant to give the same yield. These changes are due to the improvements in upstream processing. Now the challenge is that you have the same amount of product coming out of a smaller bioreactor.”
Dr. Linz mentioned several key points of intervention to meeting this challenge, including switching to a flow-through format (so that more product can be managed in a non-batch scheme) or timing batch-based process steps differently. He cited the Protein A step in antibody purification as an example, stating that Protein A is a batch approach.
“What people are now doing is running two columns—eluting one column while loading another one,” Dr. Linz noted. “From a time perspective, then, you are using the resin, in a different way. You can use one large column and process two or three runs per batch over it, or you can run it in parallel with smaller columns. This already represents an intensification of the process.”
Dr. Linz suggested that another method of intensification would be to change the operational mode of chromatography to a flow-through approach, focusing on the contaminants and “not on the product.”
“If you have a high-flow-rate unit operation, the product flows through the device, and the contaminants will be adsorbed on charged membranes or resins, getting rid of contaminants while you are purifying the product,” he elaborated. “This requires optimization, however, to select the best flow rate based on the processing time at which you are aiming.”
In older facilities, he observed, “It sometimes took five days for downstream processing. Today, it’s down to three, and through process intensification, it can be reduced even further. One impact of intensification is scheduling of your unit operations and changeover time optimization by using single-use components. Lengthy cleaning procedures and utilization of high volumes of water for injection are avoided by single-use approaches. Changeovers between different products are also benefiting from ready-to-use flow path and devices.”
According to Dr. Linz, biomanufacturing is more open to change than it has been in the last 20 years as there is an increased need for cost optimization and greater flexibility. “We have made these processes more effective on the upstream side,” he pointed out, “but now it’s up to downstream operations to catch up. The process will have a lot of challenges.”
For the time being, single-use approaches are the first step that allows manufacturing agility and lower cost of goods. The approach is particularly useful in multiproduct facilities where rapid changeovers between products are required. Further process intensification will demand greater optimization of throughput per downstream unit.



