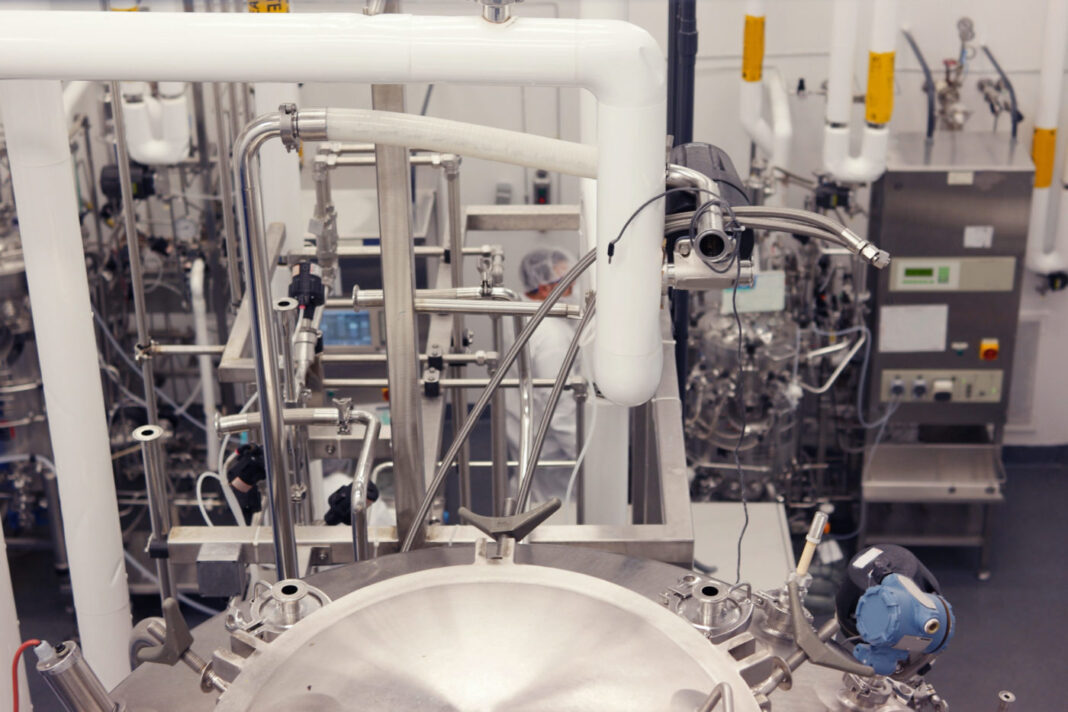
Sanofi Framingham, which opened two years ago, was designed with key ingredients of Biopharma 4.0: connecting manufacturing with R&D, paperless and data-driven operations, and intensified/flexible continuous processing.
The plant opening added to Sanofi’s presence in and around Framingham, while strengthening the company’s ability to transfer technology to other locations currently undergoing digitalization.
“The deployment of digital technologies and integration in Framingham has been a key part of our strategy to rapidly introduce new products into commercial manufacturing and has been extremely successful,” notes Larry Yudiski, head of new product introduction-technical transfer at Sanofi.
Moreover, the pandemic did not affect Sanofi’s global priorities. If anything it strengthened them since, according to Yudiski, those digital initiatives “have progressed and enabled the flexibility required to navigate the challenges of COVID-19.”
One capability noted in Sanofi’s original announcement of the new facility was technology transfer, an oft-cited explanation for project delays. Sanofi’s experience in technology transfer served as the model for such activities out of the new plant.
“To date, our technology transfers have been from the technical and development organizations into Framingham,” Yudiski explains. “For these transfers we have leveraged similarities in systems and technology from the pilot facilities to our commercial facilities. Our biggest consideration has been to utilize common technologies and digital systems between pilot and commercial sites.
“Having similar process and digital platforms at our pilot facilities has facilitated smooth technology transfer. We anticipate that this will also be a key aspect in transfers between our production facilities when we ultimately undertake such transfers.”
In Framingham, Sanofi has focused on upstream operations for its initial implementation of continuous processing. At this stage, Yudiski says, it’s possible to achieve “a dramatic improvement in scale and inter-batch consistency when compared to traditional batch processing. In principle, the level of difficulty is no different between upstream and downstream.”
Continuous processing carries special requirements for data collection, and becomes critical for ensuring process control and consistency.
“The infrastructure and qualification for these systems is important to ensure data integrity, but the real-time nature and sheer volume of data open tremendous possibilities for predictive technologies to optimize product efficiency and product quality,” Yudiski tells GEN.
Yudiski was mum on which vendors/suppliers it may be working with on either continuous processing or its IT requirement. The company does share information, however, through industry forums.
“We are also working with various regulatory agencies to continue to facilitate the development and regulatory acceptance of emerging technologies,” he says.