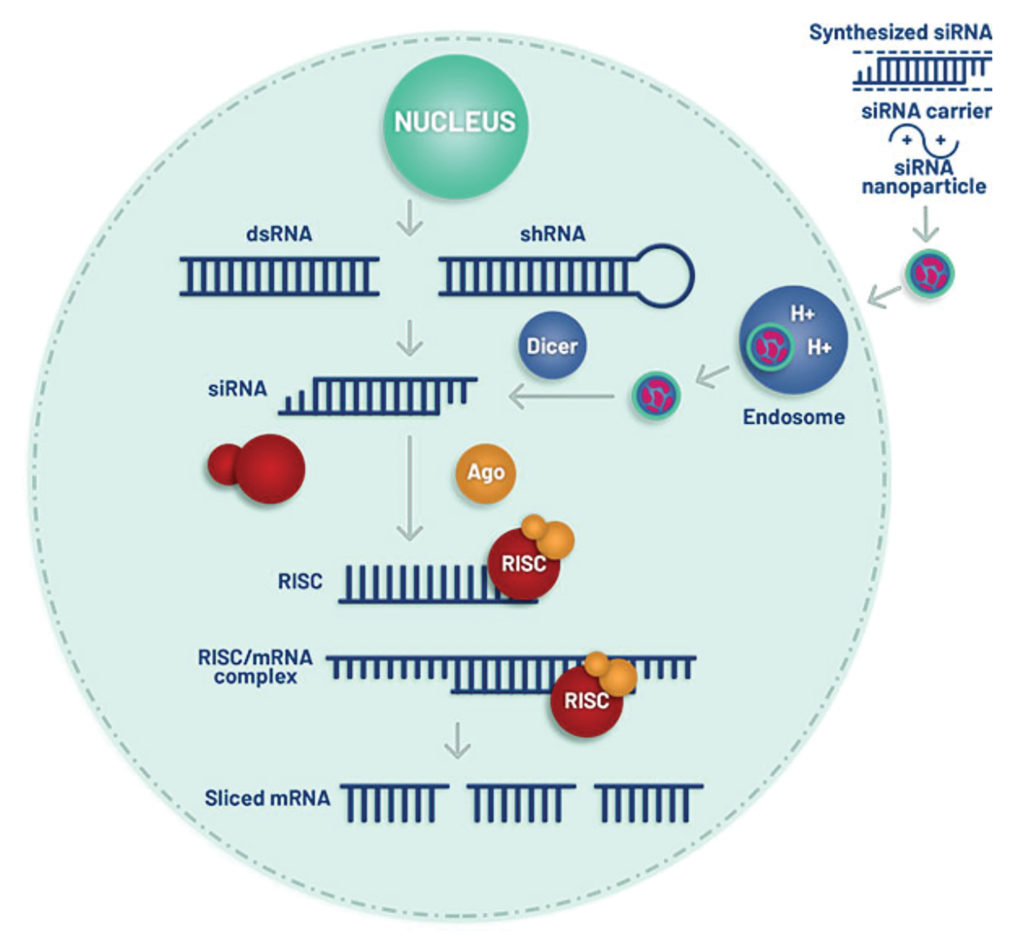
A highly conserved defense mechanism in eukaryotic cells is starting to play offense. This mechanism is a kind of RNA silencing called RNA interference (RNAi). It evolved to protect host genomes against invasive elements, such as retroviruses and transposons, and it works by silencing genes at the post-transcriptional level, that is, by degrading mRNA molecules in a sequence-specific way. RNAi, scientists have learned, may also be directed against endogenous genes.
Originally, scientists exploited RNAi as a research tool, knocking down selected genes to understand their function. Increasingly, however, scientists are relying on RNAi as a therapeutic modality. They are finding ways to trigger RNAi so that it suppresses genes associated with disease. In recent years, RNAi therapeutics have been developed to fight medical conditions such as hypertension, fibrosis, neurodegenerative diseases, metabolic conditions, and malignancies.
Reducing cholesterol levels
“We believe that our technology provides an opportunity to reimagine the treatment of hypercholesterolemia,” says John Maraganore, PhD, CEO of Alnylam Pharmaceuticals. Hypercholesterolemia, a medical and public health concern of global relevance, and a well-established risk factor for many chronic diseases, is associated with high levels of low-density lipoprotein (LDL) cholesterol that are, in many patients, hard to control with dietary changes or existing drugs.
A promising approach to new drugs is to target proprotein convertase subtilisin/kexin type 9 (PCSK9), which binds the LDL receptor and targets it for lysosomal degradation. “For many patients, the PCSK9 inhibitor approach becomes a very attractive option,” maintains Maraganore. PCSK9 inhibitors have shown an over 50% reduction of LDL-cholesterol in clinical trials.
Alnylam, in partnership with Novartis, has developed an RNAi-based PCSK9 inhibitor called inclisiran. “With inclisiran, treatment involves dosing every six months,” asserts Maraganore. “That is a game changer for treating hypercholesterolemia because nonadherence is common with existing medicines.” In fact, various studies have revealed that about 50% of patients prescribed statins are nonadherent after one year.
According to clinical trials, Inclisiran has a superior safety profile. “In the setting of hypercholesterolemia, safety is critical,” says Maraganore. “We are very pleased that the side effect profile looks very comparable to that for a placebo, and that the only common effects are injection site reactions.” He emphasizes that these effects have a low incidence and are mild in nature.
Treating pancreatic cancer
Israeli drug development company Silenseed has an RNAi-based pancreatic cancer drug in a Phase II randomized trial called PROTACT (Precise RNAi for Obtaining Targeted Cancer Therapy). PROTACT is being carried out at nine sites, four in the United States and five in Israel, says Amotz Shemi, PhD, Silenseed’s CEO, and it has enrolled patients that have stage 3 locally advanced unresectable pancreatic cancer.
The Phase II trial is evaluating Silenseed’s siG12D-LODER (LOcal Drug EluteR), which performed well in an open-label, multicenter, single-dose, Phase I study in patients with inoperable, locally advanced pancreatic cancer. The earlier trial, in which siG12D-LODER was administered in combination with standard chemotherapy, showed that the candidate drug had a high safety profile.
For reasons that are not completely understood but presumably have something to do with delayed diagnosis, the worldwide incidence and mortality of pancreatic cancer have been dramatically increasing over the last two decades. “About half of the patients are not aware of having pancreatic cancer until it becomes metastatic,” notes Shemi.
The rapid progression and high metastatic potential of pancreatic ductal adenocarcinoma have been correlated with the activity of the mutated KRAS oncogene. According to Silenseed, KRAS mutations have been considered undruggable. Yet Silenseed company is optimistic that mutated versions of KRAS can be silenced via the company’s RNAi therapeutic strategy.
LODER uses a biopolymeric scaffold that contains anti-KRASG12X (X = D, V, A, C, R, S) siRNA, which is released over 12–16 weeks in a controlled manner from miniature biodegradable capsules inserted into the tumor every three months. This novel therapy and administration route address three major challenges in cancer therapeutics: 1) therapeutic molecules need to be very specific; 2) drug concentrations within the tumor need to be high; and 3) local release needs to be lasting.
“A preliminary analysis of the tumor response and overall survival indicates that our participants do much better than what historical data shows,” Shemi asserts. “We will announce these findings later in 2020.”
Dual targeting of the cancer microenvironment
“If we select the right targets, the technology to deliver siRNA inhibitors can help us change the tumor microenvironment,” says Patrick Y. Lu, PhD, president and CEO of Sirnaomics. “siRNA drugs can go wherever monoclonal antibodies can go and even beyond.”
Sirnaomics, which is based in the United States and runs subsidiaries in China, has clinical programs focused on oncology and fibrosis. The company has developed a proprietary polypeptide nanoparticle delivery vehicle that contains a branched histidine lysine polymer and shows tropism for activated endothelial cells, which are the cell types specifically overexpressed in the new vasculature of inflammation-affected fibrotic tissue. “This polymer,” asserts Lu, “can deliver nucleic acids through an intracellular receptor that is overexpressed on those activated endothelial cells.”
A major effort in the company’s oncology program focuses on cholangiocarcinoma, the second most common liver cancer. This malignancy often develops in patients with primary sclerosing cholangitis, a condition that starts with bile duct inflammation and subsequent fibrosis. “Treatment is very difficult partly because the fibrotic tissue makes it difficult for the drug to reach the tumor cells,” notes Lu. “A drug that can resolve the fibrosis problem and at the same time treat the malignancy would be ideal.”
Sirnaomics’ scientists discovered that concomitantly targeting TGF-ß1 and Cox-2 can induce apoptosis of activated fibroblasts, cells that include tumor-associated fibroblasts, which are prevalent in the tumor microenvironment and promote malignant transformation. As TGF-ß1 overexpression in the tumor microenvironment prevents T-cell infiltration, downregulating its activity can enhance T-cell infiltration into the tumor.
“Overexpression of Cox-2 in the tumor microenvironment slows down the T cells,” explains Lu. “Downregulating Cox-2 helps the T cells mount a more vigorous attack against the tumor-related cell types.”
This work led to the development of Sirnaomics’ two lead compounds, STP705 and STP707, which have the same targets and are designed for local and systemic administration, respectively. STP705 is currently in a Phase II trial for nonmelanoma skin cancer.
“In the second quarter of 2020,” says Lu, “we expect to initiate the liver oncology study, which will include hepatocellular carcinoma, cholangiocarcinoma, pancreatic cancer, and colon cancer that has metastasized to the liver.”
Similarly, the Sirnaomics antifibrosis pipeline contains local and systemic therapeutics. The local administration product targets keloid, a fibrous nodule that forms during scar healing, and the systemic product targets primary sclerosing cholangitis.
“We are planning to start clinical trials for these therapeutics in the third quarter of 2020,” says Lu. Another compound developed at Sirnaomics, STP778, uses siRNAs to perform a dual blockade against TGF-ß1 and PD-1. “This will be widely used in various animal models,” says Lu. “We have partial data showing its efficacy and safety profile.”
A shared feature of the products in the Sirnaomics pipeline is the use of a two-pronged target approach. “Dual targeting,” insists Lu, “is the most meaningful approach, especially for oncology applications.”
Addressing more cell types
“RNAi is just entering its golden age,” says Christopher Anzalone, PhD, CEO and president of Arrowhead Pharmaceuticals. To facilitate the design of RNAi therapeutics, the company has developed proprietary algorithms that trigger the RNAi mechanism and instigate rapid, deep, and durable knockdown of target genes. RNAi therapeutics are ideally suited for targeting conditions that cannot be targeted with small molecules and biologics.
The company asserts that its technology allows lead products to be translated into the clinic in less than two years. “That is extraordinarily important,” Anzalone stresses. “It allows us to bring these essential medicines into the clinical setting.”
Besides emphasizing speed, the company is working to expand the number of tissues that RNAi therapeutics can target. At present, the usual target is the liver, which is certainly an important target. But targeting other tissues would help address many unmet medical needs. “We have been keenly interested in moving RNAi delivery to other tissues,” insists Curt Bradshaw, PhD, CSO of Arrowhead.
Different tissues require different silencing molecules. To design these molecules, Arrowhead draws on its portfolio of RNA trigger structures and chemistries. Combining these molecular elements in different ways allows the company to optimize each drug candidate on a target-by-target basis and obtain the most potent RNAi trigger.
Because the molecules used for RNAi are large and highly charged, they may have difficulty crossing membranes. This difficulty can be reduced with Arrowhead’s technological knowhow, which reflects the company’s growing comfort and experienced with RNAi delivery. “We realize this may become quite an interesting advantage,” says Bradshaw.
Advances in the RNAi space have been facilitated to a great extent by several layers of specificity for RNAi delivery, both in terms of the genes that are targeted and with respect to the cell type and cell population that the molecules are designed to enter. Many of these solutions need to be very specific to individual cell types. “There is no one-size-fits-all solution,” advises Bradshaw. He notes, however, that if Arrowhead figures out what modifications a molecule needs to target a given cell population, the exercise opens the possibility of manipulating any genes in that cell population.
In the RNAi space, Arrowhead has pioneered potential treatments for hypertriglyceridemia. The company is the sole owner of two clinical programs that are intended for this metabolic dysregulation. They’re called ARO-APOC3 and ARO-ANG3.
“These products have different profiles and will likely have different indications,” Bradshaw points out. “But both products address high triglyceride levels.”
Arrowhead has also developed the first clear cell renal cell carcinoma tumor targeting program. It has yielded a drug candidate, ARO-HIF2, that inhibits the production of HIF-2a, which is linked to tumor progression and metastasis and is overexpressed in most clear cell renal cell cancers. “We will also have the first lung program and muscle targeting program by the end of this year,” predicts Anzalone.
RNAi therapeutics are still maturing, but they have already shown promise in drug development programs, particularly with respect to safety and toleration profiles. “That is great news for patients and also for regulators, who are becoming increasingly comfortable with the modality,” says Anzalone.
Collaborating with confidence
“Our proprietary RNAi-inducing drug candidate molecules contain an extended region that enables us to apply chemistry to improve their properties,” says Douglas M. Fambrough, PhD, president and CEO of Dicerna Pharmaceuticals. “This modification-friendly region, he continues, “will be particularly important in non-liver tissues, where targeting is more complicated.” Scientists at Dicerna initially optimized the company’s proprietary GalXC™ technology for delivery to the liver, and this platform has resulted in 3 drug candidates that are currently in clinical trials and 14 more that are in early-stage development.
“RNAi delivery to the liver is now mature,” states Fambrough. “Our next set of goals includes optimizing central nervous system delivery.” One of the major efforts at Dicerna involves a collaboration with scientists at Eli Lilly & Co. to develop RNAi therapies targeting neurodegeneration. “We are now able to start approaching diseases that in the past didn’t have a good entry point,” asserts Fambrough.
In addition to the in-house programs, Dicerna recently initiated five major research and development collaborations. With Novo Nordisk, the company is targeting cardiometabolic diseases; with Eli Lilly & Co., it is targeting cardiometabolic diseases, neurodegenerative disorders, and chronic pain; with Boehringer Ingelheim, it is targeting nonalcoholic steatohepatitis; with Roche, it is targeting hepatitis B; and with Alexion Pharmaceuticals, it is targeting rare complement-mediated diseases.
“These collaborations are very large, and they were all developed in a little over two years,” Fambrough points out. “They illustrate the comfort level that the industry now has in RNAi and the remarkable interest that the technology brings.”

