August 1, 2018 (Vol. 38, No. 14)
Agilent Technologies’ Quantitative Analysis Method Incorporates a Cellular Bioenergetics Platform
Immune cells are some of the most dynamic cells in the body. They can up- and downregulate cellular processes to meet the critical and multifaceted functions of surveillance, activation, proliferation, cytokine production, antibody secretion, and pathogen clearance. Even though these functions can be analyzed and characterized with a vast array of methods (such as flow cytometry, gene expression profiling, and measurement of cytokine production), laboratories still struggle to interrogate the dynamic and rapid nature of immune cell biology in real time.
To capture functional shifts in real time, laboratories are adopting another technique: the monitoring of energy metabolism. This technique not only complements established methods, it also enables a better understanding of the immediate to early functions of immune cells.
Changes in cellular metabolism reflect metabolic reprogramming—a novel and unique marker—and occur on the order of minutes to allow changes in cell function. These metabolic changes may not only contribute to cell function changes, they may also be sufficient to cause these changes. Moreover, these metabolic changes provide a valuable set of pathway targets for modulating immune cell response, function, and fate.
The need to study the real-time kinetic responses is especially relevant in neutrophil biology. Neutrophils are phagocytic cells that represent the main antimicrobial defense of the innate immune response, and like most immune cells, neutrophils quickly ramp up glycolysis to meet their rapidly changing cellular energetic and metabolic demands.
Glucose metabolism is also important to sustain the pentose phosphate pathway that generates nicotinamide adenine dinucleotide phosphate (NADPH), one of the substrates of the NADPH oxidase (NOX2) enzyme and necessary for neutrophil extracellular trap release. Upon stimulation, the membrane-associated NOX2 is activated, resulting in a rapid and powerful oxidative burst, during which a large amount of oxygen is consumed to generate superoxide and concomitant other reactive oxygen species (ROSs). Generation of ROSs is critical for effective antimicrobial immunity and inflammatory response.
Established assays to detect and quantify oxidative bursts are indirect and based on fluorescent/luminescent detection of ROSs derived from superoxide anion formation. Despite good sensitivity, these methods are not specific, are prone to artifacts, are sensitive to the compartmentalization of the probe, and do not allow proper understanding of the duration and inactivation phase of the response, since the oxidized probe irreversibly accumulates during the assay.
An alternative method employs the Agilent Seahorse XF Analyzer, a powerful tool that not only quantifies the immediate metabolic reprogramming of neutrophils but also quantifies the oxygen consumption rate associated with NOX2 enzyme activity and ROS production. This method is not compromised by secondary ROS production, competing reactions, or probe accessibility—factors that often confound other methods.
Neutrophils Rapidly Increase Oxygen Consumption Rate upon Activation
Human peripheral blood neutrophils (huPBNs) were isolated from fresh whole blood and plated at 4 × 104 cells per well in Cell-Tak™-coated XFe96 cell culture microplates. Changes in the oxygen consumption rate (OCR) were measured in response to activation by phorbol myristate acetate (PMA)—a potent activator of the NOX2 enzyme—in the presence of mitochondrial inhibitors rotenone and antimycin A (Rot/AA).
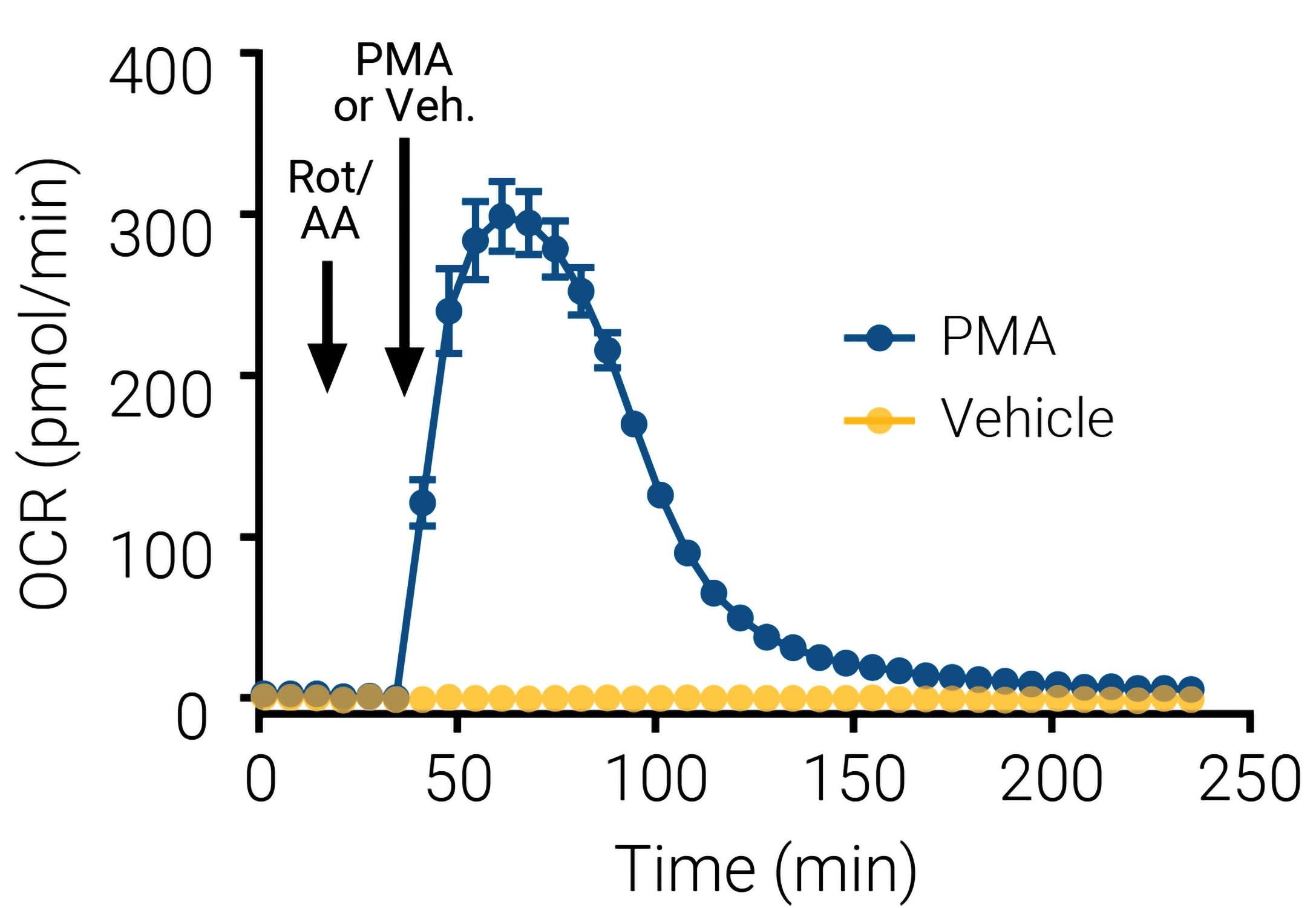
Addition of mitochondrial inhibitors in the assay before neutrophil activation ensures any oxygen consumption from mitochondrial respiration is excluded. Figure 1 shows the rapid rise in OCR observed when neutrophils are stimulated with PMA, reaching a peak of OCR within 30 min, and declining after 60 min to slowly reach the basal values 90–120 min after stimulation.
Figure 1. This XF neutrophil activation assay kinetic trace shows that oxygen consumption is an early measure of neutrophil activation. Notice how the oxygen consumption rate (OCR) changes in the presence of mitochondrial inhibitors rotenone and antimycin A (Rot/AA). Initially, 0.5 µM of each inhibitor was injected to eliminate the mitochondrial contribution to OCR. This was followed 24 min later by an injection of an activator, 100 ng/mL phorbol myristate acetate (PMA), or vehicle control (black arrows).
Activation Kinetics Quantified Using Oxygen Consumption Rate
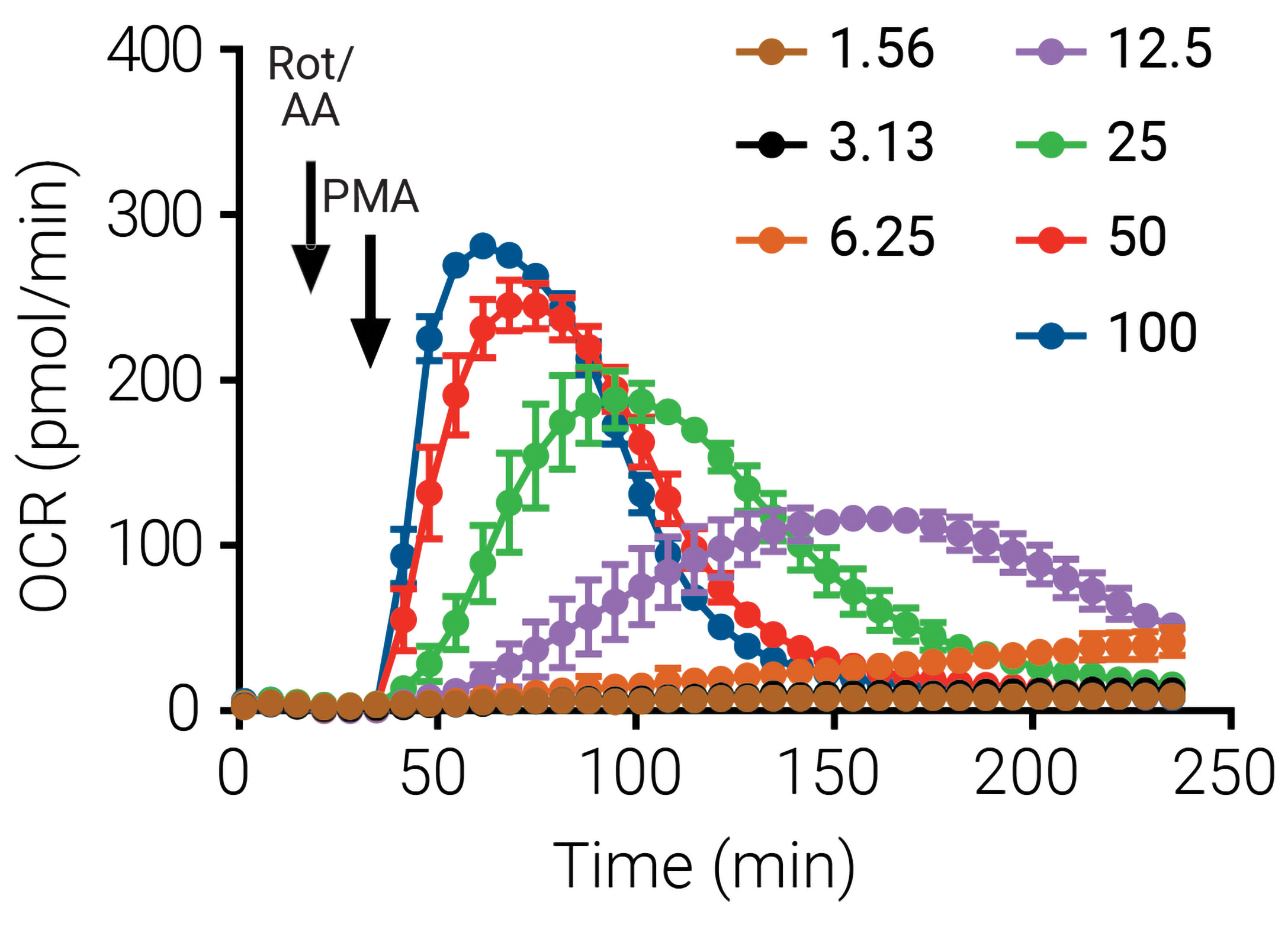
Current methods for quantifying neutrophil activation are typically end-point measurements that fail to provide a dynamic and contextual view of this process. In the next series of experiments, activation data were obtained using the XF analyzer while varying the concentration of the activating compound PMA. HuPBNs were plated at 4 × 104 per well of XF96 cell culture microplates and activated with the indicated concentrations of PMA in the presence of Rot/AA. The real-time OCR data obtained with varying doses of PMA are shown in Figure 2.
Figure 2. These XF neutrophil activation kinetic traces show real-time OCR data that were obtained when different doses of the activator PMA were administered. (Human peripheral blood neutrophils were plated at 4 × 104 per well of XF96 cell culture microplates in the presence of Rot/AA.) Such data may lead to a better understanding of the mechanism and time-course of neutrophil activation, and contribute to the development of novel agents that maintain activation at a level that allows suppression of infection while preventing inflammatory responses.
Oxidative Burst in Neutrophils Requires Glycolysis
What fuels this rapid increase in free radical production? Recent evidence suggests that glycolysis is required to support the activation of neutrophils. Glycolysis is measured in parallel for every XF assay, and as a result, this assay provides a novel view into the requirements of this cellular energy pathway during activation. The process of glycolysis results in acidification of the cell culture media, which is measured here as the proton efflux rate (PER). The increase in OCR or oxidative burst after activation with PMA is associated with a simultaneous increase of PER indicative of the dependence of neutrophils on glycolysis during activation.
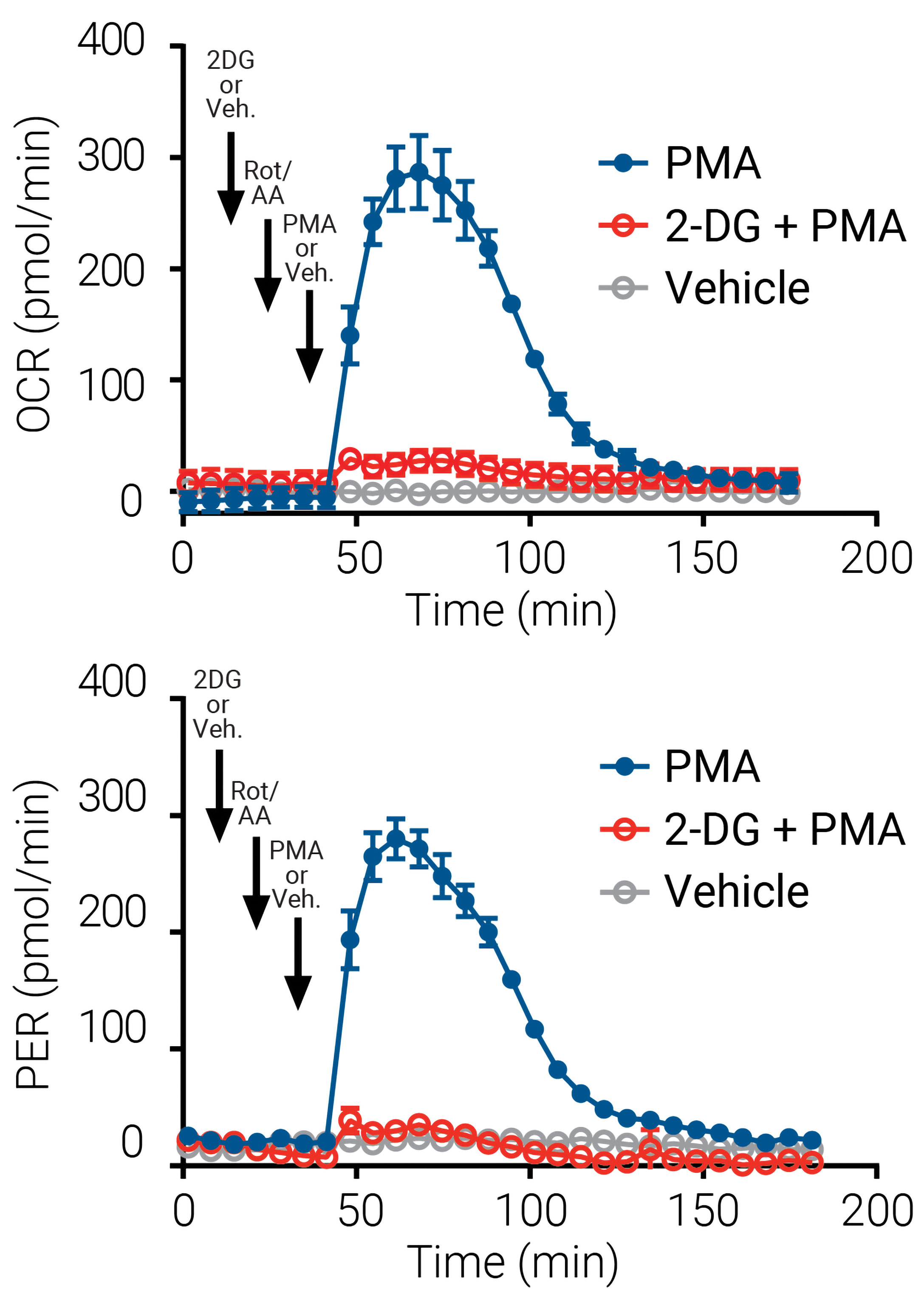
The specificity and functional relevance of the associated activation with glycolysis to meet the energy demand of oxidative burst was validated using the inhibitor 2-deoxy-d-glucose (2-DG). As shown in Figure 3, 2-DG (or vehicle), Rot/AA, and PMA (or vehicle) were serially administered, and OCR and PER were monitored. The immediate increase in OCR with PMA treatment was blocked when glycolysis was inhibited by pretreatment with 2-DG. There is an immediate parallel increase in PER with PMA treatment, and this increase in PER is likewise inhibited with 2-DG.
Figure 3. In neutrophils, an oxidative burst requires glycolysis. An XF neutrophil activation kinetic trace of OCR (top) and an XF neutrophil activation kinetic trace of proton efflux rate (PER) (bottom) in the presence of the inhibitor 2-deoxy-d-glucosesaz (2-DG). Notice that 2-DG (or vehicle), Rot/AA, and PMA (or vehicle) were serially admistered (black arrows). 2-DG (intial): 50 mM; Rot/AA (6 min later): 0.5 µM; PMA (24 min later): 100 ng/mL. Human peripheral blood neutrophils were plated at 4 × 104 per well.
Denouement
Neutrophil activation with PMA treatment causes an immediate increase in OCR, which can be monitored in real time using XF technology. This is indicative of the assembly and activation of NOX2 enzyme pathway and ROS production in activated neutrophils. There is a simultaneous increase in PER indicative of the increase in glycolysis to meet metabolic demands. Due to the noninvasive nature of the XF analyzer, downstream assays (such as PCR assays, ELISAs, and neutrophil extracellular trap assays) may be performed on the same cells. This application is a specific, kinetic assay performed on live cells in real time, and it provides temporal resolution of neutrophil activation that is not possible with other assays.
For Research Use Only. Not for use in diagnostic procedures.



