Sponsored content brought to you by

The fight to improve cancer therapy has included the development of ligand-mediated active targeting to guide anticancer drug cargos more directly to their specific targets. Tumor-associated macrophages (TAMs), found in the tumor microenvironment, mostly express an anti-inflammatory “M2” phenotype and contribute to tumor progression, angiogenesis, and metastasis. Researchers at University of Washington have developed and further refined peptide-targeted drug delivery aimed at selectively depleting TAMs. To do this, they have fine-tuned the sequence of a targeting peptide, M2pep, designed to deliver a conjugated drug cargo directly to the M2 macrophage. Modifications to the peptide sequence improved serum stability and also increased M2 macrophage binding activity.
Peptides That Guide Drugs to the Target with Precision
Efforts to increase the efficacy and reduce side effects of cancer therapies have included developing methods to deliver drugs to their target with higher precision. One approach is ligand-mediated active targeting, which involves conjugating the drug molecule to a guiding ligand molecule that recognizes the overexpressed receptor in the tumor and promotes cellular internalization to boost the potency of therapy. While antibodies, as well as proteins and oligonucleotides, have shown promise as targeting ligands, peptides have significant advantages, especially over antibodies. The small size of peptides means high tumor penetration, low immunogenicity, and high scalability. Phage display screening enables rapid screening, and the diversity of amino acid functional groups provides a lot of flexibility in tailoring the targeting peptide and its conjugation to the drug cargo. However, peptides do have disadvantages that include lower target affinity and serum stability due to degradation by proteases; but these problems may be overcome by multivalent display of the peptides and rational sequence modifications, respectively.
A Rational Approach to Improve the Targeting of Tumor-Associated Macrophages
Chayanon Ngambenjawong, together with colleagues at the department of bioengineering and the Molecular Engineering and Sciences Institute at University of Washington had already developed a targeting peptide. This work involved using phage display biopanning to identify a peptide (M2pep) that binds preferentially to murine anti-inflammatory M2 macrophages/M2-like TAMs. M2pep has been conjugated with a pro-apoptotic peptide, KLA, to mediate selective depletion of M2-TAMs in a syngeneic CT-26 colon carcinoma model. The therapeutic effect was, however, severely reduced by the short serum half-life and low target-binding affinity of M2pep. The team‘s next objective has been to improve the stability and affinity through rational peptide engineering of M2pep using strategies that include N-terminal acetylation, D-amino acid substitution, and peptide stapling/macrocyclization.
Using a PS3™ Peptide Synthesizer (Gyros Protein Technologies), the team synthesized a succession of M2pep variants, guided by progressive insights into structure/function of various sections of the sequence. An overview of some of these sequences is shown in Table 1. The team tested the stability of the sequences in normal mouse serum, monitoring degradation with MALDI-TOF mass spectrometry, and also tested binding against M1 and M2 macrophages. Finally, biodistribution was tested in two carcinoma models, CT-26 and 4T1.
Modifying Terminal and Internal Sequences Reduces Degradation
The team started by modifying the sites susceptible to peptidases. N-terminus acetylation reduced exolytic degradation, but endolytic degradation at positions W10/W11 and S16/K17 was still an issue.
In modifying the peptide internally, the team had to take into account an important M2pep receptor-binding motif, DPWXXXXW (see Table 1). Modifying W10 to a D-tryptophan (W10w) reduced degradation but also reduced binding. W10P, W10Y, or K9R substitutions did not improve stability of W10/W11, but modifying the trilysine spacer to D-lysines improved the stability at positions S16/K17.
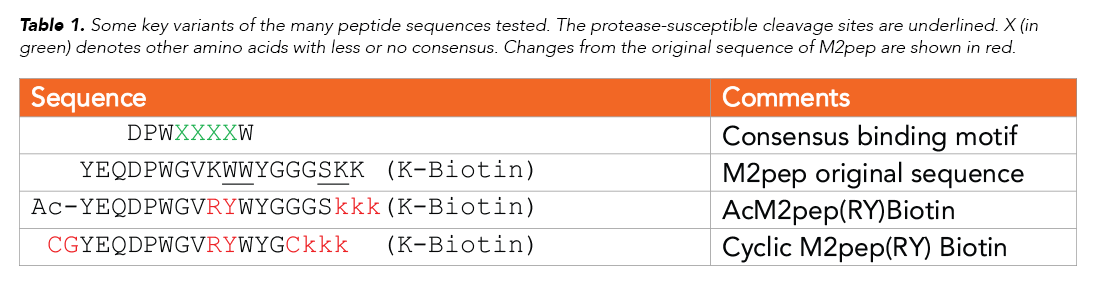
A K9R analog was tested—since K9 is the only lysine in the targeting region and replacing this with arginine would produce an AcM2pep with a C-terminal lysine that would simplify the conjugation to drug cargos with an activated NHS ester. Among the variety of tested modifications (see Reference for complete list), K9R and W10Y substitutions showed increased binding activity.
Cyclization Does the Trick
Knowing that head-to-tail cyclization of peptides can improve structural rigidity, bioactivity and serum stability, the team modified the linear N-terminal acetylated peptide with two flanking cysteines to enable disulfide cyclization. The resulting peptide had substantially higher serum stability. The linear acetylated form could be degraded within 2–8 h while the cyclic form, which had comparable binding activity, was still detectable after 48 h.
In Vivo Studies Confirm Efficacy
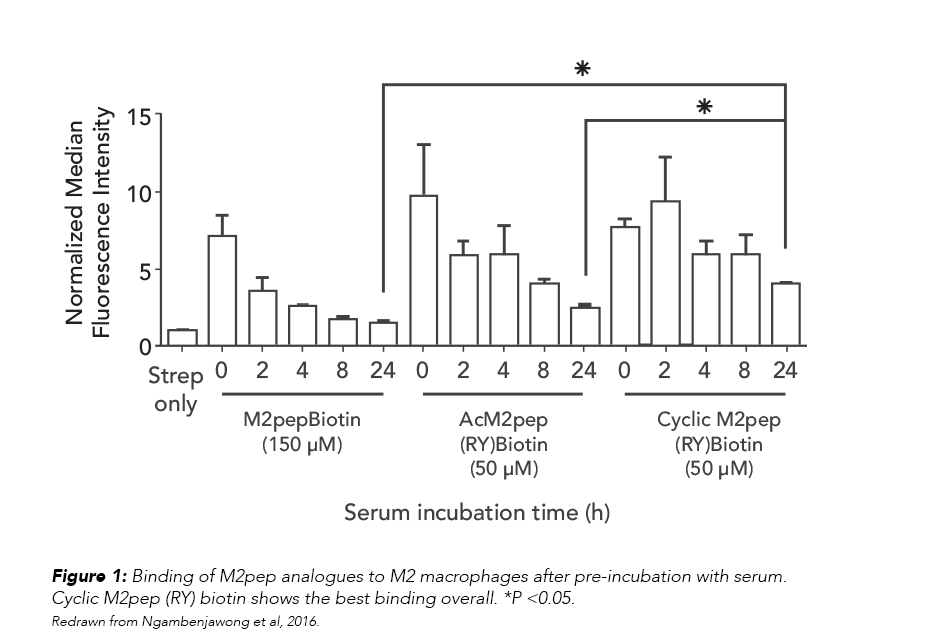
Targeting TAMs means that M2pep analogs will be exposed to serum during systemic circulation, so the team tested the effect of serum exposure on the analogs’ binding to macrophages. The relative binding activities of the analogs, which corresponded to their serum stability, were in the following order: cyclic M2pep(RY)biotin > AcM2pep(RY)biotin > M2pepbiotin (Figure 1).
Finally, the team investigated the in vivo accumulation of analogs, using the CT-26 and 4T1 carcinoma models to show that cyclic M2pep(RY) outperformed M2pep in both tumor localization and selective accumulation in M2-like TAMs.
Conclusions
The researchers at the University of Washington have clearly demonstrated that a rational design approach enhanced the targeting of tumor-associated M2 macrophages, which resulted in an improved cyclic analog of M2pep. This approach can be applied to other types of tumors and M2 macrophage-related disease. Finally, the authors suggest that future studies could include further stability improvements by incorporating azide- and alkyne-functionalized amino acids to cyclize via CuAAC.
Reference
Serum stability and affinity optimization of an M2 macrophage-targeting peptide (M2pep).
Ngambenjawong C et al. Theranostics. 2016 Jun 15;6(9):1403-14. doi: 10.7150/thno.15394. eCollection 2016.
Cyf Ramos-Colon, Ph.D. ([email protected]), is a senior scientist at Gyros Protein Technologies.


