March 15, 2010 (Vol. 30, No. 6)
High-Frequency Ultrasound and MicroMarker Contrast Agents Can Provide Critical Information
Angiogenesis, the formation of new blood vessels from existing vessels, is an aggressively studied process, especially in cancer research. One of the most commonly referred to angiogenic pathways involves vascular endothelial growth factor and its receptors. Numerous potential therapeutics or experimental procedures are being tested for their ability to alter the angiogenic pathways crucial to the growth of tumors. There are various types of tumor models that are of interest, including transgenic, orthotopic, and subcutaneous, all of which are well suited for imaging using ultrasound.
Ultrasound imaging is a noninvasive, real-time imaging technique that allows for longitudinal studies on the same animal. Imaging sessions provide anatomical and functional data and, with the use of contrast agents, molecular data as well.
VisualSonics’ Vevo® 2100 High Resolution Ultrasound Imaging System provides axial resolution down to 30 µm. This type of resolution allows for the detection of tumorigenesis well before the lesion is palpable. MicroMarker™ contrast agents can be used to assess vascularity and molecular expression of specific targets in vessels down to the capillary level.
Ultrasound imaging is noninvasive, therefore, the same tumor can be studied over the course of an experiment, leading to stronger data and requiring fewer animals to get significant results.
Materials and Methods
The Vevo 2100 High Resolution Ultrasound Imaging System was used to acquire all of the images in this study. Images were acquired from athymic nude mice implanted with hepatocarcinoma cells subdermally in the hind limb four to five weeks before imaging.
Nontargeted MicroMarker contrast agents were used to enhance the visualization of blood flow down to the capillary level. The contrast agents were injected intravenously, typically through the tail vein, and circulated freely through functional blood vessels. The microbubbles were 2–3 µm in size and were made up of a phospholipid shell containing a polyethylene glycol outer shell, along with a perfluorobutane/nitrogen gas core.
A bolus injection of the nontargeted MicroMarker contrast agents provides a wash-in curve for analysis (Figure 1). Here, two regions of interest were drawn on the tumor—the purple outlining the entire tumor and the pink outlining an area with increased resistance to perfusion. Software analysis tools allow for quantification of the peak enhancement, which is a measure of relative blood volume, and for quantification of the time to peak, which is a measure of relative blood velocity.
In Figure 1, the highly resistive area shows a much longer time to peak, therefore, blood is moving into that area at a much lower velocity than the whole tumor, the peak enhancement is also much lower, indicating a lower blood volume when compared to the whole tumor.
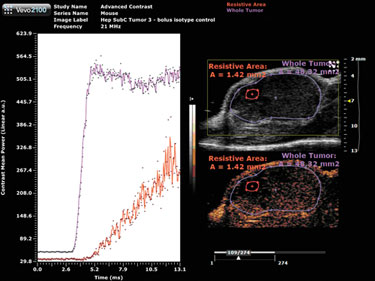
Figure 1. MicroMarker contrast agent imaging
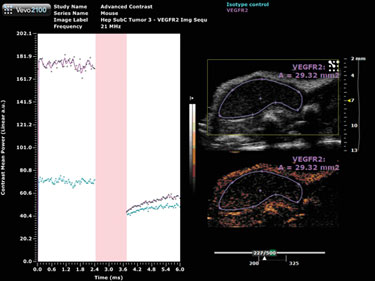
Target-ready MicroMarker contrast agents are similar in structure to the non-targeted MicroMarker contrast agents, however, there is a streptavidin molecule attached to the polyethylene glycol (PEG) molecule that makes up the outermost layer of the shell. This molecule allows for the conjugation of any biotinylated molecule to the microbubble, such as a primary antibody for vascular endothelial growth factor receptor 2 (VEGFR2). The conjugated antibody, for example, can then bind to its ligand on the surface of endothelial cells.
A negative control, in the form of an isotype control antibody, is utilized to allow for the quantification of any non-specific binding of the contrast agent.
A destructive pulse must be applied in order to perform the quantification; a destruction curve can then be plotted for both the isotype control and VEGFR2 antibodies (Figure 2). Quantification involves calculating the change in contrast enhancement following the destruction pulse. The results from this study show some nonspecific binding, however, there is substantial specific VEGFR2 binding in this tumor model.
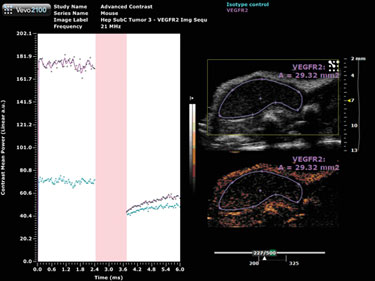
Figure 2. Target-ready MicroMarker contrast agent imaging
Conclusions
The images and techniques described in this article show the utility of the Vevo 2100 High Resolution Ultrasound Imaging System as a tool for in vivo imaging and quantification of tumor angiogenesis. The noninvasive nature of ultrasound imaging allows the same tumor to be studied over the course of an experiment, leading to much stronger data and requiring fewer animals to get significant results.
The use of nontargeted MicroMarker contrast agents provides information on the vascularity of the tumor down to the capillary level. Molecular information about the expression of specific endothelial cell surface targets, such as VEGFR2, can be obtained by using the target-ready MicroMarker contrast agents.
The techniques discussed allow for acquisition of real-time data on actively perfused vessels, and therefore, would easily allow for the study of acute or chronic changes in perfusion or expression of specific markers in response to a potential therapeutic or experimental procedure.
Tonya Coulthard is applications scientist, and Catherine Theodoropoulos, Ph.D. ([email protected]), is director, product applications at VisualSonics. Web: www.visualsonics.com.